Novel bivalent spermine-based neutral neogalactolipids for modular gene delivery systems†
E. A. Ivanovaa,
A. V. Filatovb,
N. G. Morozovaa,
M. A. Zenkovab and
M. A. Maslov*a
aLomonosov Moscow University of Fine Chemical Technologies (MITHT), 86 Vernadskogo ave., Moscow, 119571 Russian Federation. E-mail: mamaslov@mail.ru; Fax: +7 4959368901; Tel: +7 4959368903
bInstitute of Chemical Biology and Fundamental Medicine SB RAS, 8 Lavrentieva ave., Novosibirsk, 630090, Russian Federation. E-mail: marzen@niboch.nsc.ru; Fax: +7 3833635153; Tel: +7 3833635160
First published on 26th October 2015
Abstract
New bivalent spermine-based neutral neogalactolipids have been synthesized to develop effective modular gene delivery systems (MGDS) targeting hepatocyte asialoglycoprotein receptors. MGDS composed of new neogalactolipids, cationic gemini-amphiphile, and DOPE were agglutinated in the presence of lectin RCA120 and provided efficient delivery of fluorescently-labeled oligodeoxyribonucleotides into HepG2 cells.
One of the problems of gene therapy is the development of stable and safe non-viral transport systems for the delivery of therapeutic nucleic acids (NA) into the target cells with efficiency equal to that of viral vectors. Using cationic liposomes as delivery systems is one of the promising approaches for gene therapy.1,2 Positively charged complexes composed of cationic liposomes and NA (called “lipoplexes”) are able to adsorb on to the plasmatic membranes of cells and enter them by means of an endocytic pathway.3 However, the wide application of cationic liposomes is limited by their low efficiency and unspecific NA delivery into target cells.4 These drawbacks are related to the presence of different biological barriers for lipoplexes such as the instability in biological fluids, the interaction with blood serum proteins and plasmatic and nucleic membranes, and endosomal degradation. A concept of modular gene delivery systems (MGDS) was introduced ten years ago as an appropriate structural paradigm for synthetic non-viral vector.5–8 In MGDS, nucleic acid is condensed into cationic liposomal formulations consisting of different lipophilic modules that mediate environmental and cellular receptor interactions and intracellular trafficking to overcome limiting barriers.
Genetic damages of liver cells lead to serious diseases such as hemophilia, hypercholesterolemia, cancer and other.9,10 It is known that hepatocytes express the asialoglycoprotein receptors (ASGPr), which recognize D-galactose or N-acetyl-D-galactosamine terminal residues;11,12 therefore, the targeting of therapeutic NA to hepatocytes can be achieved by MGDS containing neogalactolipids (neo means non-natural) as a targeting module. ASGPr as a multidomain binding protein exhibits significantly different affinities for ligands depending on their structures. ASGPr bind monovalent ligands (free D-galactose, lactose, and simple galactosides) with millimolar dissociation constants (Kd ∼ 10−3 M), which are far below those needed to achieve glycotargeting under physiological conditions. In comparison, bivalent galactose-terminated oligosaccharides (compounds with two carbohydrate ligands) bind with affinities that are three-orders-of-magnitude higher (Kd ∼ 10−6 M).13 To construct multivalent carbohydrate-containing conjugates suitable for multiple molecular interactions with ASGPr, a number of different polyfunctional core molecules [tris(2-aminoethyl)amine, tris(hydroxymethyl)-aminomethane, pentatreitole derivatives, heterocyclic bases, amino acids, oligopeptides, and carbohydrates14–19 are used.
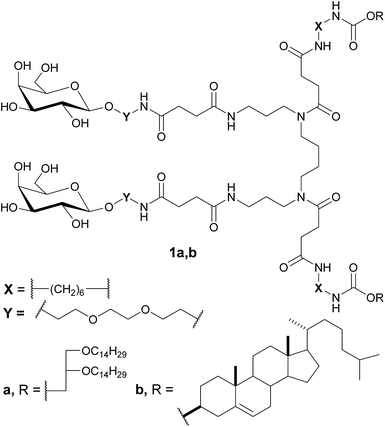
Previously, to develop MGDS targeted to the hepatocytes, we designed and synthesized bivalent galactose-containing neutral lipids on the basis of L-glutamic acid as core molecule.20 These neoglycolipids were successfully incorporated into liposomal formulations composed of polycationic gemini amphiphile (as the condensing module) and zwitterionic lipid DOPE (as the helper module). Specific binding of galactose-decorated liposomes was demonstrated using the ricin agglutination test. Here we described a convenient approach for the synthesis of novel bivalent spermine-based neutral neogalactolipids 1a,b (Fig. 1) as well as physicochemical characteristics and biological activity of targeted MGDS composed of them.
Natural polyamines, spermine and spermidine, play an important role in the vital activity of cells and can pack DNA to toroidal and rod-like structures. Lipophilic derivatives of polyamines condense DNA more efficiently than natural polyamines and can be used as non-viral NA delivery systems. On the basis of spermine, various amphiphilic molecules were synthesized to deliver oligonucleotides, DNA and siRNA into eukaryotic cells.21–26 Also it was found that HepaRG cells (progenitor cells which are capable to differentiate into hepatocyte-like cells) could be easily transfect by cationic liposomes composed of lipophosphonates, lipophosphoramidates and betaine-type lipids.27
To construct spermine-based neutral neogalactolipids 1a,b, cholesterol and 1,2-di-O-tetradecyl-rac-glycerol were used as hydrophobic domains for the incorporation of neogalactolipids into a liposomal bilayer. Hexamethylene and triethylene glycol spacers were chosen to distance the galactose moieties from hydrophobic domain and to provide flexibility and independent orientation of carbohydrate units in the space.
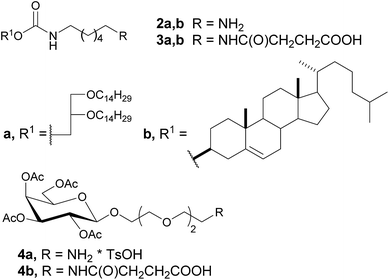
For the synthesis of neogalactolipids 1a,b, sets of building blocks, hydrophobic 2a,b and 3a,b and carbohydrate 4a,b precursors, which contain various spacers and terminal functional groups (Fig. 2), were prepared. Compounds 2a,b and 3a,b with terminal amino or carboxyl groups were synthesized as previously described.20 Carbohydrate precursor 4b was obtained by the treatment of galactoside 4a20 with succinic anhydride in the presence of Et3N in 69% yield.
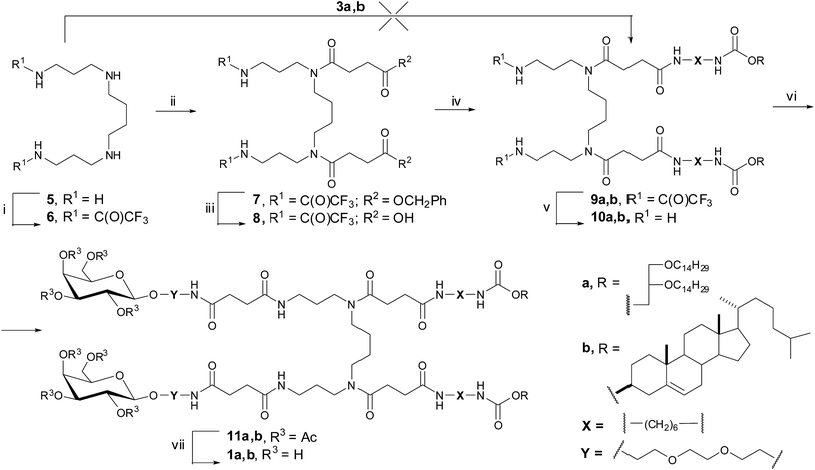
Due to the symmetry of spermine (5) and the presence of four functional amino groups, different arrangement of hydrophobic and carbohydrate domains can be achieved. We suggested to introduce firstly the hydrophobic precursors 3a,b on spermine secondary nitrogen atoms followed by the connecting of glycoside 4b on primary amino groups (Fig. 3). Spermine (5) was treated with ethyl trifluoracetate to give bis-amine 6 in 95% yield. It is known that the synthesis of N4,N9-diacylspermines can be done by an acylation of the protected spermine derivatives with fatty acids in the presence of DCC and HOBt.28 Another effective approach utilizes acid chlorides or mixed anhydrides as acylating agents. Chloroanhydrides and mixed anhydrides29 of compounds 3a,b were prepared. Unfortunately, the treatment of bis(trifluoroacetamide) 6 with these anhydrides led to the formation of cyclic amide of compounds 3a,b but not to the desired products 9a,b. The condensation of compounds 3a,b and 6 in the presence of HBTU, N-methylmorpholine, and HOBt30 was also ineffective. Therefore, for the synthesis of neogalactolipids 1a,b, a new approach was implemented, which involved the initial introduction of succinic residue into spermine molecule 6 and subsequent condensation with hydrophobic precursors 2a,b containing a terminal amino group.
Bis(trifluoroacetamide) 6 was treated by the chloride of succinic acid monobenzyl ester31,32 in the presence of catalytic amount of DMAP at 0 °C for 60 min. After column chromatography on silica gel, spermine derivative 7 was obtained in 37% yield. Benzoyl protective groups were removed by catalytic hydrogenation on Pd/C in the presence of an excess of ammonium formate to give compound 8 with terminal carboxyl groups. Further coupling of hydrophobic components 2a,b and compound 8 was carried out in the presence of HBTU and DIPEA in anhydrous DMF to afford compounds 9a,b in 76% and 90% yields, respectively. After the unblocking of primary amino groups in conjugates 9a,b by sodium hydroxide in methanol spermine derivatives 10a,b, containing two hydrophobic domain were obtained. The attachment of galactoside 4b to conjugates 10a,b promoted by HBTU led to the formation of di-substituted glycoclusters 11a,b in 43% and 32% yields, respectively. The deacetylation of compounds 11a,b under the Zemplen reaction condition yielded the desired bivalent neutral neogalactolipids 1a,b in 72% and 90% yields, respectively. The structures of the synthesized neogalactolipids 1a,b were confirmed by 1H and 13C NMR spectroscopy as well as 1H,1H-COSY 2D-NMR spectroscopy and mass spectrometry (see ESI†).
Targeted MGDS composed of polycationic lipid 2D3,33,34 zwitter-ionic lipid DOPE, and neogalactolipid 1a (2.5%, 5%, and 10% mol) were prepared as previously described.34 The particle size and ζ-potential measurements were carried out to assess the influence of neoglycolipid molar ratio on the physicochemical characteristics of MGDS (Table 1). All targeted MGDS were positively charged (ζ-potentials are within the range +45 to +50 mV) and characterized by monomodal size distribution. The increasing of neogalactolipid 1a amount in liposomal formulations from 0% to 10% resulted in the dramatical decrease of the average particle diameters from 114 nm to 44 nm. Additionally, the PDI values also decreased, indicating the stabilizing effect of compound 1a on liposomal formulations.
To estimate the accessibility and affinity of galactosyl ligands exposed on MGDS surface toward ASGPr, a ricin agglutination test was performed. Ricinus communis lectin (RCA120) promotes the aggregation of liposomes that exhibit D-galactose moieties on their surface.35 The addition of RCA120 (1 mg mL−1) to targeted MGDS L1, L2, and L3 resulted in the increasing of dispersion optical density and the precipitation of liposomes (Fig. 4). Conventional liposomes L0 were unaffected. The agglutination level depended on the molar ratio of neogalactolipid 1a into liposomes. When solution of free D-galactose (100 mg mL−1) was added to a mixture of L3 and RCA120, a dramatic decrease of the optical density of the solution was observed, indicating the binding specificity of galactose-containing MGDS toward RCA120.
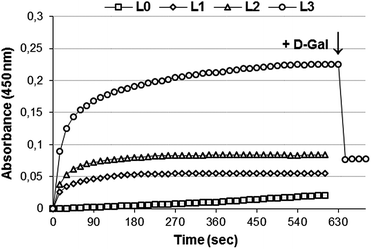 | ||
| Fig. 4 Agglutination of the targeted MGDS containing neogalactolipids 1a after the addition of RCA120: square – L0; rhomb – L1; triangle – L2; circle – L3 liposomes. | ||
To estimate the cytotoxic effect of MGDS, HEK 293 cells were incubated with the liposomes at concentrations ranging from 5 to 80 μM for 24 h under the serum-free conditions, and MTT test36 was performed. It was found that all the studied MGDS were not toxic for cells; at concentration 80 μM, the IC50 values are not achieved (see ESI†).
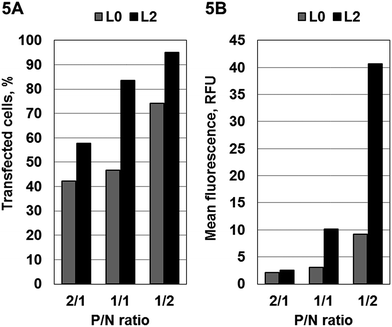
To estimate the capability of the targeted MGDS to transfer NA into eukaryotic cells, the uptake of a 21-mer 5′-fluorescein isothiocyanate-labeled oligonucleotide (FITC-ODN) mediated by one of the targeted (L2) and conventional (L0) liposomes was studied (Fig. 5). Transfection was performed using HepG2 cells, which overexpressed ASGPr, and the efficiency of FITC-ODN accumulation was determined by the percentage of fluorescent cells (Fig. 5A) in the population and fluorescence intensity of cells (Fig. 5B).
Complexes between MGDS and FITC-ODN were formed at various P/N (phosphate to nitrogen) ratios (2/1, 1/1 and 1/2) and incubated with cells within 4 h. A number of FITC-positive HepG2 cells as well as fluorescence intensity values were affected by the presence of neogalactolipids 1a in the MGDS and by the P/N ratio (Fig. 5). Only liposomes L2 mediate cellular delivery of FITC-ODN at reasonably high levels, which 1.3–1.8 fold exceeds the one observed for conventional liposomes L0 (Fig. 5A). Moreover, at P/N = 1/2 the observed accumulation of FITC-ODN mediated by targeted MGDS L2 was significantly higher than that of liposomes L0, which lacked addressing component (Fig. 5B). Typical images (see ESI†) showed the accumulation of FITC-labelled oligonucleotide in both the cytoplasm and the nucleus of HepG2 cells after 4 h of incubation with the lipoplexes composed of FITC-ODN and L2.
Conclusions
We have elaborated the synthetic route for the preparation of the new branched neogalactolipids based on spermine containing two hydrophobic and two targeting domains for the development of modular gene delivery systems (MGDS). The investigations of physicochemical characteristics of MGDS showed that the addition of neogalactolipids resulted in the stabilization of liposomal formulations and decrease their diameters twice. Ricin agglutination test indicated that the targeted MGDS obtained were bound specifically with RCA120, and the efficiency of this process depended on the molar ratio of neogalactolipids in the liposomal formulations. Low toxicity and efficient delivery of short oligonucleotides into HepG2 cells allow to consider MGDS decorated by new neogalactolipids as promising objects for further in vitro and in vivo studies.Acknowledgements
This research was supported by Russian Foundation for Basic Research (research project No. 13-04-40183 comfi and 13-04-40181 comfi).Notes and references
- Non-viral Vectors for Gene Therapy, Part 1, ed. L. Huang, M. C. Hung and E. Wagner, Elsevier, Amsterdam, 2005 Search PubMed.
- M. A. Maslov and M. A. Zenkova, in Non-viral Gene Therapy, ed. X. Yuan, InTech, Rijeka, 2011, pp. 349–380 Search PubMed.
- R. L. Juliano, X. Ming and O. Nakagawa, Bioconjugate Chem., 2012, 23, 147 CrossRef CAS PubMed.
- S. Djurovic, N. Iversen, S. Jeansson, F. Hoover and G. Christensen, Mol. Biotechnol., 2004, 28, 21 CrossRef CAS.
- L. de Laporte, J. Cruz Rea and L. D. Shea, Biomaterials, 2006, 27, 947 CrossRef CAS PubMed.
- K. Kostarelos and A. D. Miller, Chem. Soc. Rev., 2005, 34, 970 RSC.
- V. P. Torchilin, Nat. Rev. Drug Discovery, 2005, 4, 145 CrossRef CAS PubMed.
- T. Nakamura, H. Akita, Y. Yamada, H. Hatakeyama and H. Harashima, Acc. Chem. Res., 2012, 45, 1113 CrossRef CAS.
- J. Prieto, R. Hernandez-Alcoceba, G. Gonzalez-Aseguinolaza, G. Mazzolini, B. Sangro and G. Kramer, Expert Opin. Biol. Ther., 2004, 4, 1073 CrossRef CAS PubMed.
- K. Kunath, A. von Harpe, D. Fischer and T. Kissel, J. Controlled Release, 2003, 88, 159 CrossRef CAS PubMed.
- G. Ashwell and J. Harford, Annu. Rev. Biochem., 1982, 51, 531 CrossRef CAS.
- M. Spiess, Biochemistry, 1990, 29, 10009 CrossRef CAS PubMed.
- T. K. Lindhorst, in Carbohydrate-Modifying Biocatalysts, ed. P. Grunwald, CRC Press, Boca Raton, 2011, pp. 119–182 Search PubMed.
- T. K. Lindhorst, Top. Curr. Chem., 2002, 218, 201 CrossRef CAS.
- R. T. Lee, P. Lin and Y. C. Lee, Biochemistry, 1984, 23, 4255 CrossRef CAS PubMed.
- E. M. V. Johansson, E. Kolomiets, F. Rosenau, K. E. Jaeger, T. Darbrea and J. L. Reymond, New J. Chem., 2007, 31, 1291 RSC.
- I. Vrasidas, S. André, P. Valentini, C. Böck, M. Lensch, H. Kaltner, R. M. J. Liskamp, H. J. Gabius and R. J. Pieters, Org. Biomol. Chem., 2003, 1, 803 CAS.
- M. Dubber, A. Patel, K. Sadalapure, I. Aumüller and T. K. Lindhorst, Eur. J. Org. Chem., 2006, 5357 CrossRef CAS.
- D. Deniaud, K. Julienne and S. G. Gouin, Org. Biomol. Chem., 2011, 9, 966 CAS.
- E. A. Ivanova, M. A. Maslov, N. G. Morozova, G. A. Serebrennikova and V. V. Chupin, RSC Adv., 2012, 2, 4600 RSC.
- J.-P. Behr, B. Demeneix, J.-P. Loeffler and J. Perez-Mutul, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 6982 CrossRef CAS.
- F. Lamarche, M. Mével, T. Montier, L. Burel-Deschamps, P. Giamarchi, R. Tripier, P. Delépine, T. le Gall, D. Cartier, P. Lehn, P.-A. Jaffrès and J.-C. Clément, Bioconjugate Chem., 2007, 18, 1575 CrossRef CAS PubMed.
- M. Mével, N. Kamaly, S. Carmona, M. H. Oliver, M. R. Jorgensen, C. Crowther, F. H. Salazar, P. L. Marion, M. Fujino, Y. Natori, M. Thanou, P. Arbuthnot, J.-J. Yaouanc, P. Alain Jaffrès and A. D. Miller, J. Controlled Release, 2010, 143, 222 CrossRef.
- N. Niyomtham, N. Apiratikul, K. Suksen, P. Opanasopit and B. Yingyongnarongkul, Bioorg. Med. Chem. Lett., 2015, 25, 496 CrossRef CAS PubMed.
- I. A. Petukhov, M. A. Maslov, N. G. Morozova and G. A. Serebrennikova, Russ. Chem. Bull., 2010, 59, 260 CrossRef CAS.
- M. A. Maslov, T. O. Kabilova, I. A. Petukhov, N. G. Morozova, G. A. Serebrennikova, V. V. Vlassov and M. A. Zenkova, J. Controlled Release, 2012, 160, 182 CrossRef CAS PubMed.
- V. Laurent, A. Fraix, T. Montier, S. Cammas-Marion, C. Ribault, T. Benvegnu, P.-A. Jaffres and P. Loyer, Biotechnol. J., 2010, 5, 314 CrossRef CAS PubMed.
- H. M. Ghonaim, S. Li and I. S. Blagbrough, Pharm. Res., 2009, 26, 19 CrossRef CAS PubMed.
- M. M. Joullié and K. M. Lassen, ARKIVOC, 2010,(viii), 189 Search PubMed.
- P. Neuner and P. Monaci, Bioconjugate Chem., 2002, 13, 676 CrossRef CAS PubMed.
- J. Winkler, E. Urban, D. Losert, V. Wacheck, H. Pehamberger and C. R. Noe, Nucleic Acids Res., 2004, 32, 710 CrossRef CAS PubMed.
- N. S. Bodor, US Pat. 4 264 765, 1981.
- E. A. Ivanova, Y. I. Tokareva, M. A. Maslov, N. G. Morozova and V. V. Chupin, Vestn. MITKHT, 2012, 7, 54 CAS.
- O. V. Markov, N. L. Mironova, M. A. Maslov, I. A. Petukhov, N. G. Morozova, V. V. Vlassov and M. A. Zenkova, J. Controlled Release, 2012, 160, 200 CrossRef CAS PubMed.
- S. R. S. Ting, G. Chen and M. H. Stenzel, Polym. Chem., 2010, 1, 1392 RSC.
- J. Carmichael, W. G. de Graff, A. F. Gazdar, J. D. Minna and J. B. Mitchell, Cancer Res., 1987, 47, 936 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR- and mass-spectra of compounds synthesised and cytotoxicity of liposomes. See DOI: 10.1039/c5ra17389b |
| This journal is © The Royal Society of Chemistry 2015 |