Thermal stability of MnOx–CeO2 mixed oxide for soot combustion: influence of Al2O3, TiO2, and ZrO2 carriers
Abstract
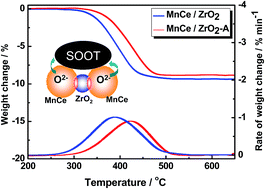
MnOx–CeO2 (Mn/Ce = 0.15/0.85) mixed oxide materials supported on ZrO2, Al2O3, and TiO2 were prepared by citrate sol–gel method to prevent the CeO2-based oxide catalyst from sintering during soot combustion reaction. The materials were characterized using XRD, BET method, HRTEM, H2-TPR, and XPS. Results indicate that the MnOx–CeO2/TiO2 catalyst is rapidly sintered at 800 °C because of the TiO2 phase change from anatase to rutile. By contrast, MnOx–CeO2 catalysts supported on Al2O3 and ZrO2 show high thermal stability. After aging at 800 °C for 10 h, the MnOx–CeO2 crystalline structure remained highly dispersed on the carrier. Particularly with the ZrO2 carrier, the amount of surface oxygen species and redox properties of MnOx–CeO2 can be well-maintained for the synergistic effect of CeO2 and ZrO2 in high temperature environments. Good agreement is found between the low-temperature redox property (200 °C to 400 °C) and the soot combustion activity of the catalysts.

 Please wait while we load your content...
Please wait while we load your content...