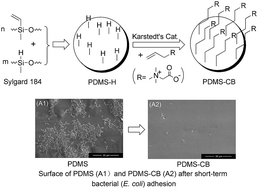
Polydimethylsiloxane (PDMS) is a widely used material for biomedical applications. In this work, a convenient method for the covalent modification of PDMS with carboxybetaine was developed and used to construct a biocompatible and anti-fouling coating. Following the preparation of a Si–H functionalized PDMS film by adjusting the molar ratio of the two components used in Sylgard 184 silicone elastomer networks, the allyl carboxybetaine (ACB) was grafted to the PDMS surface via a hydrosilylation reaction in the presence of a Karstedt's catalyst. ATR-FTIR and water contact angle measurements revealed that carboxybetaine was introduced to the PDMS surface successfully. The biocompatibilities of PDMS and carboxybetaine-modified PDMS (PDMS-CB) films were evaluated by cytotoxicity, hemocompatibility, and dynamic clotting time. The anti-fouling properties of PDMS-CB were evaluated by protein adsorption and bacterial adhesion measurements. The results showed that the carboxybetaine layer could enhance the biocompatibility of PDMS and reduce the adsorption of protein and adhesion of bacteria efficiently.