Evaluation of poly(pyrrole-2-carboxylic acid) particles synthesized by enzymatic catalysis
A. Kausaite-Minkstimieneab,
A. Ramanavicieneab,
R. Simanaitytea,
D. Gabrielaitisa,
L. Glumbokaitea and
A. Ramanavicius*cd
aDepartment of Analytical and Environmental Chemistry, Faculty of Chemistry, Vilnius University, Naugarduko str. 24, LT-03225 Vilnius, Lithuania
bDepartment of Immunology, State Research Institute Centre for Innovative Medicine, Santariskiu str. 5, LT-08406 Vilnius, Lithuania
cLaboratory of NanoBioTechnology, Department of Materials Science and Electronics, Institute of Semiconductor Physics, State Scientific Research Institute Centre for Physical Sciences and Technology, A. Gostauto str. 9, LT-01108 Vilnius, Lithuania. E-mail: arunas.ramanavicius@chf.vu.lt
dDepartment of Physical Chemistry, Faculty of Chemistry, Vilnius University, Naugarduko str. 24, LT-03225 Vilnius, Lithuania
First published on 3rd December 2015
Abstract
In this study an environmentally friendly synthesis of poly(pyrrole-2-carboxylic acid) (PCPy) particles dispersed in water–ethanol medium using enzymatic catalysis is proposed. The polymerization of pyrrole-2-carboxylic acid was initiated by the oxidant hydrogen peroxide resulting from the redox enzyme glucose oxidase (GOx) catalyzed glucose oxidation reaction. The main evidence of the polymerization process was the origin and increase of the absorption peak at 465 nm indicating the presence of PCPy oligomers. The PCPy formation rate in different pH medium was investigated and compared with the formation rate of the PCPy synthesized by chemical oxidative polymerization. The best medium for the enzymatic polymerization was determined at pH 5.0, while for the chemical method it was at pH 2.0. The GOx had a significant positive impact on the outcome of the polymerization reaction and colloidal stability of the formed PCPy particles. The GOx catalyzed polymerization reaction was faster than that based on chemical oxidative polymerization but the precipitation of insoluble precipitate was observed after a longer period of polymerization. The morphology of the PCPy particles was characterized by SEM. Additionally, the presence of carboxylic groups in the formed PCPy particles was confirmed by FTIR spectroscopy and potentiometric back-titration.
Introduction
π–π conjugated polymers (CP) are an exciting class of organic materials combining properties of metals and polymers. The modern development of these polymers began in 1977 as A. J. Heeger, A. G. MacDiarmid and H. Shirakawa developed special polymers with metal-like properties.1,2 These unconventional properties of an organic material encourage worldwide interest and numerous other CP such as polyaniline, polypyrrole (PPy) and polythiophene have been synthesized. Nowadays, CP are widely used and have numerous practical applications in biosensors,3 biomedical devices,4 enzyme immobilization matrices,5 artificial muscles6 or drug delivery.7,8 Research on CP, which could be useful for biomedical applications, expanded greatly in the 1980s when it was found that these materials are compatible with many biological molecules.9 Cell and tissue compatibility of CP was demonstrated also both in vitro and in vivo.10,11 Therefore, the synthesis of CP possessing biocompatibility properties is highly desirable, and is still a challenge.CPs are usually synthesized by electrochemical and chemical oxidative polymerization methods. The electrochemical polymerization is based on the anodic oxidation of a certain monomer in low-pH aqueous or non-aqueous solutions leading to a thin polymeric film formation on the electrode surface. Meanwhile, by chemical oxidative polymerization polymer is obtained as an amorphous suspension precipitated in bulk of the solution. In the chemical polymerization process, monomer is oxidized by chemical oxidizing agents. Since the CP have been shown to be biocompatible, there is a strong interest in improving the synthesis procedure to produce polymer in an environmentally friendly and efficient manner. The oxidative polymerization of pyrrole using environmentally friendly chemical oxidants such as hydrogen peroxide12 or a catalytic amount of iron(III) chloride13 has been reported. According to these reports, good yield of polymer was obtained only in low-pH aqueous medium. Therefore, the CP synthesis using enzymes has become more attractive as an alternative synthetic route. Horseradish peroxidase,14,15 soybean peroxidase,14,16,17 bilirubin oxidase,18 laccase19,20 and glucose oxidase21 have been used as catalysts in the synthesis of CP. Biocatalytic polymerization using enzymes is advantageous because it does not require strong acidic media or additional purification steps.22 It can be carried out in an aqueous medium near neutral pH. Thus, the enzymatic polymerization is an environmentally friendly and a very simple one-step process.
PPy is one of the most extensively investigated CP. PPy materials have received great attention in bioelectronics and biomedical application due to their inherent features, including high conductivity, outstanding stability and good biocompatibility.23 In vivo animal studies have shown that low concentrations of PPy nanoparticles (<200 μg mL−1) have very low long-term cytotoxicity.24 It has been used in biosensors as relatively stable and porous matrix for enzyme immobilization or for drug delivery as biological compatible polymer matrix in which number of drugs and enzymes can be incorporated by way of doping.25 Substitution in the pyrrole ring gives the possibility of introducing specific functional groups on the PPy chain. Similarly to the PPy, functionalized PPy may also be used over the areas mentioned above after drugs and enzymes immobilization. The presence of carboxylic groups on the polymer of poly(pyrrole-2-carboxylic acid) (PCPy) provides the possibility of covalent immobilization of biologically active molecules as bio-receptors through a covalent bond with the carboxylic functionalities, therefore the PCPy could be an electroactive material with advanced properties suitable for amperometric biosensors and drug delivery systems.
The polymerization of pyrrole-2-carboxylic acid (PCA) has not been widely investigated. In spite of the huge amount of research related to the polymerization of pyrroles, those substituted on the 2-position have received much less attention as possible monomers. This could be related to the fact that have been published many research claiming PCA decarboxylation in acidic buffered aqueous solutions.26,27 These studies argues that the rate of decarboxylation of PCA increase with increasing acidity of the medium. PCA is subject to acid catalyzed decarboxylation in strong acids but resistant to decarboxylation in less acidic solutions.28 Vandersteen et al.29 have shown that the observed rate constant for decarboxylation of pyrrole-3-carboxylic acid is about 300 times smaller than that for pyrrole-2-carboxylic acid. However, in recent years electrochemical synthesis of PCPy has been published.30 Foschini et al.31 proposed a theoretical approach to explain experimental results obtained from the electrochemical synthesis of PCPy films. According to their study, the monomer (PCA), dimers and trimers are oxidized in the C4 or C5 positions of the heterocyclic ring of the PCA structure. The monomer initially oxidizes at the electrode/solution interface after the withdrawal of an electron, yielding a cation radical with predominantly unpaired electron density in the C5 position. The propagation step involved in obtaining dimer requires two electrons per consumed monomer. The propagation steps for the formation of structures with higher conjugation lengths consume two electrons for each added monomer, following the coupling reaction in the C4 and C5 positions between oligomer and monomer.
In the present work, the chemical oxidative and enzymatic polymerization of the PCA is described and compared. According to our knowledge, neither chemical oxidative nor enzymatic polymerization has not been applied in the production of the PCPy as it has been demonstrated for the PPy.32–37 The aim of the current study was to demonstrate and investigate in detail the possibility to synthesize PCPy by using enzyme glucose oxidase (GOx) as a catalyst. Some aspects characterizing this simple enzymatic synthesis of PCA oligomers and PCPy particles are presented in this article. The advantages of this study are: (i) that the GOx is utilizing environmentally friendly substrates (β-D-glucose and dissolved oxygen) and it is producing environmentally friendly products (hydrogen peroxide and D-glucono-1,5-lactone) and (ii) that this polymerization reaction can be carried out in water–ethanol medium at ambient conditions.
Experimental
Chemicals
Glucose oxidase (GOx) from Aspergillus niger type X-S 117.2 U mg−1 enzymatic activity was obtained from SIGMA-ALDRICH Chemie GmbH (Steinheim, Germany). Pyrrole-2-carboxylic acid (PCA) was purchased from Alfa Aesar GmbH & Co KG (Karlsruhe, Germany). Hydrogen peroxide (H2O2), sodium acetate (CH3COONa), acetic acid (CH3COOH), potassium hydrogen phosphate dodecahydrate (Na2HPO4 × 12H2O), potassium dihydrogen phosphate (KH2PO4), hydrochloric acid (HCl) and potassium hydroxide (KOH) were purchased from AppliChem GmbH (Darmstadt, Germany). D-(+)-Glucose (C6H12O6) was obtained from Carl Roth GmbH & Co (Karlsruhe, Germany). Ethanol, 100%, was purchased from MERCK KGaA (Darmstadt, Germany). All commercial chemicals were of analytical grade or better, and were used as received.2.0 mol L−1 stock solution of glucose was prepared in water at least 24 h before use to reach equilibrium of α and β optical isomers. 100 mg mL−1 stock solution of GOx was prepared in a buffer solution composed of 50 mmol L−1 CH3COONa, 50 mmol L−1 Na2HPO4 and 50 mmol L−1 KH2PO4 (A-PBS), pH 6.0. 0.5 mol L−1 stock solution of PCA was prepared in ethanol. All aqueous solutions were prepared in UHQ water (conductivity less than 1 μS cm−1) purified by DEMIWA rosa 5 (WATEK, Czech Republic).
Synthesis of the PCPy
Polymerization reactions of PCA were carried out in A-PBS solutions of different pH (pH 2.0–9.0). All reactions were carried out in closed plastic disposable cuvettes (total reaction volume was 1.5 mL). All components for chemical oxidative polymerization of PCA were mixed in the following sequence: 854 μL of A-PBS (of certain pH), 600 μL PCA stock solution (0.5 mol L−1 in ethanol, freshly prepared), 46 μL H2O2 (30% solution). The initial concentrations in the polymerization solutions were 200 mmol L−1 PCA and 300 mmol L−1 H2O2. All components for enzyme catalysed polymerization of PCA were mixed in the following sequence: 735 μL of A-PBS (of certain pH), 600 μL PCA stock solution (0.5 mol L−1 in ethanol, freshly prepared), 50 μL glucose stock solution (2.0 mol L−1 in water), 15 μL GOx stock solution (100 mg mL−1 in A-PBS, pH 6.0, freshly prepared). The initial concentrations in the polymerization solutions were 200 mmol L−1 PCA, 200 mmol L−1 glucose and 1 mg mL−1 GOx. pH of all polymerization solutions were adjusted to a certain pH with CH3COOH, HCl or KOH. In both cases, the polymerization was performed out at room temperature in the dark.Study of the PCPy formation
The process of polymerization reaction was monitored by recording UV-Vis absorption spectra between 300 and 800 nm using UV-Vis spectrophotometer Perkin-Elmer LAMBDA 25 (Perkin-Elmer Inc, USA). The absorption spectra were recorded immediately after preparation of the polymerization solution and at a certain time from the start of the polymerization. The UV-Vis absorption was monitored in plastic disposable cuvettes with optical path of 1 cm.Imaging of the PCPy particles by SEM
The morphology of the formed PCPy particles was investigated by ultra-high resolution field emission scanning electron microscope FE-SEM SU-70 (Hitachi, Japan). Samples for imaging were prepared by shaking a polymerization solution and then deposition of 3 μL of such homogenised solution on atomically flat silicon wafer (CrysTech Kristall technologie, Germany). Then from this drop the solvent was evaporated at room temperature and the wafer with adsorbed sample was thoroughly washed twice using UHQ water. After washing of all residual salts and poorly adsorbed PCPy particles from the surface, the sample was dried and used for further analysis.FTIR analysis of the PCPy
Infrared absorption spectra of the synthesized PCPy in 500–3650 cm−1 wavenumber region were recorded using Perkin Elmer spectrum BX FTIR spectrometer (Perkin-Elmer Inc, USA). The samples for analysis were prepared by centrifuging of formed PCPy, washing of collected centrifugate twice with UHQ water and once again repeating of centrifugation and washing procedures. Then separated polymer was freeze-dried in Christ Alpha 2-4 LSC Freeze dryer (Martin Christ Gefriertrocknungsanlagen GmbH, Germany) and prepared PCPy powder was used for FTIR analysis. The FTIR spectra were recorded by preparing PCPy–KBr pellets using 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 mass ratio of PCPy
10 mass ratio of PCPy![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KBr.
KBr.
Potentiometric back-titration
100 mg of freeze-dried PCPy powder was mixed with a 10 mL of 50.0 mmol L−1 solution of NaHCO3 and stirred for 30 min. Then the excessive NaHCO3 was titrated with a 50.0 mmol L−1 solution of HCl, and the solution pH was monitored by a Mettler Toledo SevenEasy pH meter. Control experiment was also carried out by titration a 10 mL of 50.0 mmol L−1 solution of NaHCO3 with the same 50.0 mmol L−1 solution of HCl. The difference of the HCl solution volumes at the equivalent points between these two titration curves was the evidence of the presence of carboxylic groups in the formed PCPy particles.Results and discussion
Despite of active research related to the polymerization of various pyrroles, the polymerization of pyrrole-2-carboxylic acid (PCA) has not been widely investigated. To our best knowledge, poly(pyrrole-2-carboxylic acid) (PCPy) has not been synthesized in water–ethanol medium neither chemical oxidative nor enzymatic polymerization methods. In this work, the PCPy particles were synthesized by an enzyme catalyzed and chemical oxidative polymerization technique using H2O2 as an oxidant. In our current study we take an advantage of an enzymatically by glucose oxidase (GOx) produced H2O2 to polymerize aniline and pyrrole in a broad pH region of medium.12,22,38–40 The GOx is an oxidoreductase that catalyzes the oxidation of β-D-glucose to H2O2 and D-glucono-1,5-lactone using molecular oxygen as an electron acceptor. During this process D-glucono-1,5-lactone is non-enzymatically hydrolysed to gluconic acid.An enzyme catalyzed polymerization solutions were based on four major compounds: the PCA – polymerisable monomer, the GOx – H2O2 producing enzyme, the glucose – reducing substrate of the GOx, and dissolved oxygen – oxidizing substrate of the GOx. The initial concentrations in the polymerization solutions were 200 mmol L−1 PCA, 200 mmol L−1 glucose and 1 mg mL−1 GOx. The process of PCA polymerization reaction was monitored by recording the UV-Vis absorption spectra between 300 and 800 nm. The changes of the absorption spectra as a function of reaction time were followed. The spectra recorded at a certain time from the start of the polymerization are shown in Fig. 1A for the reaction taking place in solution at pH 3.0, and in Fig. 1B for the reaction taking place in solution at pH 5.0. Initially all polymerization solutions were slightly yellowish and only two low intensity absorption peaks of the GOx at 359 and 445 nm were present in the absorption spectra (Fig. 1, start). The H2O2, which was produced during catalytic reaction of the GOx, was able to oxidize the PCA in the initiation step resulting in chemically active cation radicals of the monomer and polymerization solutions turned to yellowish brown colour due to the polymerization process. The rate of changes of solution colour was depended on the pH of polymerization solution.
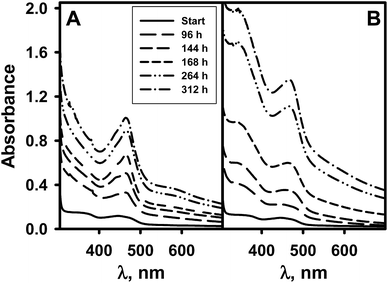 | ||
| Fig. 1 Absorption spectra of the PCPy oligomers in A-PBS–ethanol solution pH 3.0 (A) and pH 5.0 (B) containing 200 mmol L−1 PCA, 1 mg mL−1 GOx and 200 mmol L−1 glucose. | ||
The main evidence of polymerization process was the origin and increase of absorption peak at 465 nm indicating the presence of the pyrrole ring in the formed PCA oligomers or soluble isolated nanoparticles of the PCPy.41–44 As can be seen from the Fig. 1, different absorption spectra for the PCPy synthesized in polymerization solutions of different pH during the polymerization reaction were observed. Only one absorption peak at 465 nm was observed for the PCPy synthesized at pH 2.0, 3.0 and 4.0. When the polymerization was carried out between pH 5.0 and pH 9.0 two absorption peaks at 345 and 465 nm were followed. The absorption peak at 345 nm corresponds to the π–π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond resulting from the formation of the PCPy. The absorption peak at 465 nm corresponds to a bipolaron transition, a characteristic feature for the oxidized state of the PCPy. In addition, the absorption peak at 465 nm can be attributed to the higher conjugation length of the PCPy. It is noted that the position of absorption peaks depend on various factors such as counter ions, solvent, chemical structure and the morphology of the polymer.45 In the case of the PCPy synthesized in polymerization solution at pH from 5.0 to 9.0, the peak intensity at 345 nm is stronger compared with that at 465 nm. Such effect can be explained by the fact that the PCPy is at lover level of oxidation in less acidic polymerization solutions. In this study observed absorption peaks for the PCPy is in a good agreement with previous reported for the PPy.35,46
C double bond resulting from the formation of the PCPy. The absorption peak at 465 nm corresponds to a bipolaron transition, a characteristic feature for the oxidized state of the PCPy. In addition, the absorption peak at 465 nm can be attributed to the higher conjugation length of the PCPy. It is noted that the position of absorption peaks depend on various factors such as counter ions, solvent, chemical structure and the morphology of the polymer.45 In the case of the PCPy synthesized in polymerization solution at pH from 5.0 to 9.0, the peak intensity at 345 nm is stronger compared with that at 465 nm. Such effect can be explained by the fact that the PCPy is at lover level of oxidation in less acidic polymerization solutions. In this study observed absorption peaks for the PCPy is in a good agreement with previous reported for the PPy.35,46
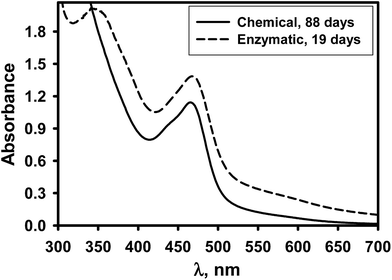
The process of the GOx catalyzed PCA polymerization reaction was compared with the chemical oxidative polymerization process by recording the UV-Vis absorption spectrum between 300 and 800 nm with increasing reaction time. For this reason, polymerization of the PCA was carried out in polymerization solutions composed of necessary components and at pH values ranging from 2.0 to 9.0. The initial concentrations of polymerization solutions used in the monitoring of chemical oxidative polymerization process were 200 mmol L−1 of PCA and 300 mmol L−1 of H2O2. The initial concentrations of polymerization solutions used in the monitoring of enzyme catalyzed polymerization process were 200 mmol L−1 of PCA, 200 mmol L−1 of glucose and 1 mg mL−1 of GOx. The experimental data presented in Fig. 2 illustrates the absorption spectra recorded in the polymerization solutions with pH 5.0. As shown, only one absorption peak at 465 nm was registered for the PCPy synthesized by chemical oxidative polymerization method. Similar spectra were registered for other analyzed pHs of polymerization solutions.
Results presented in Fig. 1 and 2 show a steady increase in absorbance at 345 and 465 nm over the entire range of the absorption spectrum with increasing polymerization time. This phenomenon is related to light scattering by the PCPy particles dispersed in polymerization solution. It was reported that by chemical oxidative polymerization synthesized oligomers with polymerization degree up to 30 monomers are soluble47 and polymerization takes place in the liquid phase. Oligomers with greater degree of conjugation are insoluble. Insoluble oligomers formed solid insoluble particles and then the polymerization continues mainly on the surface of these polymeric particles. Eventually the PCPy particles precipitate from the polymerization solution as insoluble black powder.
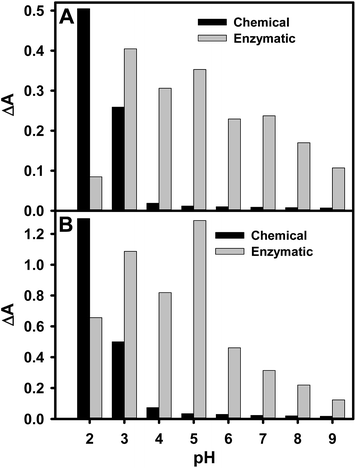
The formation rate of PCPy particles synthesized by the GOx catalyzed polymerization was compared with the formation rate of the particles synthesized by chemical oxidative polymerization. Results presented in Fig. 3 show relationship between absorbance of the PCA oligomers or soluble isolated nanoparticles of the PCPy and the pH value of polymerization solution. In order to eliminate the influence of light scattering by the PCPy particles dispersed in polymerization solution on the registered absorbance of the PCA oligomers or soluble isolated nanoparticles of PCPy, the absorbance value at 465 nm wavelength determined by oligomers or soluble isolated nanoparticles were calculated as a difference between absorbance at 465 and 800 nm (ΔA = Aλ465 − Aλ800). Absorbance of the PCA oligomers or soluble isolated nanoparticles of the PCPy synthesized by chemical oxidative polymerization was registered within 88 days period. It is clearly seen that the best medium for chemical oxidative PCA polymerization was strongly acidic medium. The highest ΔA value, and thus the highest PCPy formation rate were observed at pH 2.0 (Fig. 3). An increase in the pH of the medium resulted in a decrease in the rate of formation of the PCPy, and polymer formation was almost undetectable when the pH of polymerization solution exceeded pH 6.0. The same effect has been reported for the PPy synthesized by chemical oxidative polymerization methods.12,48 The experimental studies show that the polymerization reaction was very slow. At pH 2.0 and 3.0, precipitation of insoluble black precipitate “pyrrole black” occurred within 7 and 20 days of polymerization reaction, respectively; while in the less acidic medium the polymerization solutions turned to yellowish brown colour without visible precipitation within 88 days.
According to results obtained for the GOx catalyzed polymerization, the formation of PCPy was observed in the entire investigated pH range. A similar effect has been reported for the GOx catalyzed polymerization of pyrrole and aniline.22,36 Significant polymerization rate differences detected with and without GOx, demonstrated a high impact of enzyme to PCA polymerization rate. According to our experimental data the highest ΔA values at six-day period were observed at pH 3.0 (Fig. 3). However, in a subsequent polymerization period the highest ΔA values were recorded at pH 5.0. These results can be explained by the facts that the polymerization takes place faster in acidic medium, but free glucose oxidase from Aspergillus niger type X-S exhibits maximal activity at pH 5.5.49 Therefore, at pH 5.0 GOx exhibited the maximal catalyzed oxidation of β-D-glucose to H2O2 and D-glucono-1,5-lactone reaction rate and the highest stability. The enzymatically produced H2O2 in a broad pH region of polymerization solution was able to oxidize the PCA in the initiation step of polymerization resulting in chemically active cation radicals of the monomer with predominantly unpaired electron density in the C5 position as proposed by Foschini et al.31 Then these cation radicals couple through deprotonation, forming soluble dimers. In the propagation step, dimers are oxidized again, and further coupling leads to the formation of soluble trimers and then the formation of soluble structures with higher conjugation lengths, following the coupling reaction in the C4 and C5 positions between oligomer and monomer.
According to accomplished studies the enzyme catalyzed polymerization reaction was faster than in the case of chemical oxidative polymerization. However, the precipitation of insoluble black precipitate was observed after a longer period of polymerization. At pH 2.0, 3.0, 4.0 and 5.0, precipitation of insoluble black precipitate occurred within the 9, 23, 30 and 38 days of polymerization reaction, respectively. In the less acidic medium the polymerization solutions turned to yellowish brown colour without visible precipitation within 88 days. As can be seen from the Fig. 3, the calculated ΔA values are not correlated with the sedimentation rate. It could be explained that the GOx has a positive impact on the outcome of the polymerization reaction and colloidal stability of the formed PCPy particles. It allowed us to make prediction that the particles of PCPy, which were under critical size, were suspended in the polymerization solution. It is in agreement with the results illustrating that particles of conjugated polymers might be soluble in the aqueous medium.50–54 Moreover, it might be predicted that during the GOx catalyzed β-D-glucose oxidation reaction pH gradient locally decreased near to the active site and the surface of enzyme, because of gluconic acid formed, while H2O2 gradient locally increased. Acidic medium and high concentration of oxidizing agent are the most optimal conditions for the polymerization of conjugated polymers.55 It is likely that under such conditions the PCA polymerization begins to take place and resulting PCPy encapsulated or at least partially by PCPy layer covered glucose oxidase. This approach may be well suited for the incorporation other biomolecules into resulting polymer.
The polymerization of PCA was confirmed using FTIR spectroscopy. In order to get reliable FTIR data formed PCPy particles were separated from not polymerized pyrrole-2-carboxylic acid according to the protocol described in experimental part and briefly reported here: after a defined polymerization reaction time, the PCPy particles obtained by chemical oxidative polymerization (at pH 2.0 and by the GOx catalyzed polymerization at pH 3.0 and 5.0) were separated from the polymerization solution by centrifuging and twice repeated washing of them with water; then separated polymer was freeze dried and prepared PCPy powder was used for FTIR spectroscopy based analysis (sample was prepared in the form of KBr pellets). Fig. 4 show FTIR spectra of PCPy prepared by chemical oxidative (C) and enzyme catalyzed polymerization at pH 2.0 (D) and 5.0 (E). For comparison, we also synthesized PPy in A-PBS–ethanol solution, pH 2.0, by chemical oxidative polymerization, using solution with 200 mmol L−1 of pyrrole and 300 mmol L−1 of H2O2. The PPy FTIR spectrum is shown in Fig. 4A. The PCA FTIR spectrum is shown in Fig. 4B. All spectra have very similar infrared absorption peaks to those described in other studies related to the PPy formation.56–62 The FTIR spectra of PCA (Fig. 4B) and PCPy (Fig. 4C–E) additionally show infrared absorption peaks corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups that are absent in the PPy spectrum, indicating the presence of carboxylic groups63,64 in the formed PCPy. Thus, the spectroscopic evidence confirms that the PCPy was produced during the chemical oxidative and enzyme catalyzed polymerization reaction. The infrared absorption peaks of C
O and O–H groups that are absent in the PPy spectrum, indicating the presence of carboxylic groups63,64 in the formed PCPy. Thus, the spectroscopic evidence confirms that the PCPy was produced during the chemical oxidative and enzyme catalyzed polymerization reaction. The infrared absorption peaks of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups and their respective assignments are presented in Table 1.
O and O–H groups and their respective assignments are presented in Table 1.
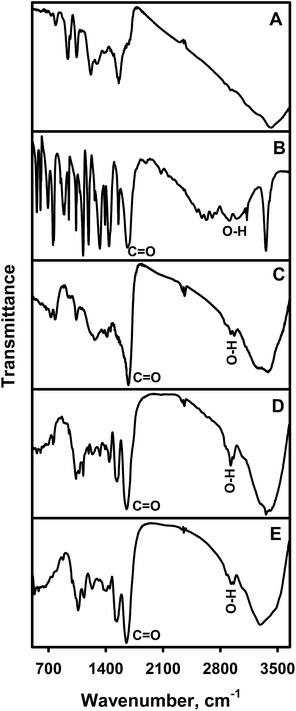 | ||
| Fig. 4 FTIR spectra of PCA (B), PPy (A) and PCPy prepared by chemical oxidative polymerization at pH 2.0 (C) and enzyme catalysed polymerization at pH 2.0 (D) and pH 5.0 (E). | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups and their respective assignments
O and O–H groups and their respective assignments
| Assignment | Infrared absorption peak position, wavenumber (cm−1) | |||
|---|---|---|---|---|
| PCA | PCPy, chemical | PCPy, enzymatic, pH 2.0 | PCPy, enzymatic, pH 5.0 | |
Stretching C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
1664 | 1674 | 1652 | 1652 |
| Stretching O–H | 2912 (broad) | 2970 | 2926 | 2945 |
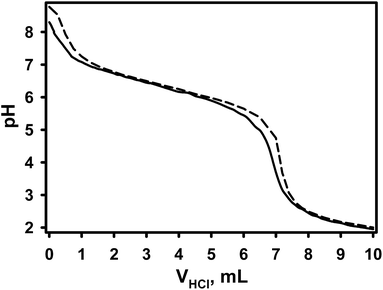
In order to validate the presence of carboxylic groups in the formed PCPy particles a potentiometric back-titration was performed. In the present study 100 mg of freeze-dried PCPy particles were mixed with NaHCO3 solution. Then the excessive base was titrated with HCl solution. The titration curve of PCPy particles is shown in Fig. 5 (solid line). The control experiment was carried out by titration known amount of NaHCO3 solution with the HCl solution (Fig. 5 (dashed line)). As can be seen from (Fig. 5), by the addition of HCl the pH of solution decreases accordingly and the equivalent points were well-defined by the sharp decrease of the solution pH with the addition of about 6.90 (solid line) and 7.15 mL (dashed line) of HCl. The difference of used HCl volumes at the equivalent points between these two titration curves evidenced the presence of carboxylic groups in the formed PCPy particles.
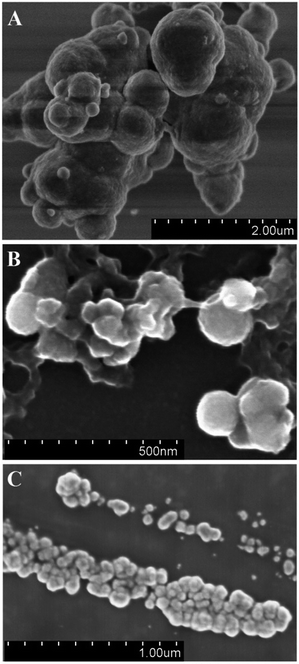
The morphology of the synthesized PCPy particles was characterized by SEM. The SEM images revealed a globular morphology for the PCPy particles prepared both by chemical oxidative (Fig. 6A) or enzyme catalyzed polymerization (Fig. 6B and C). Virtually no difference in particle surface morphology was observed. The SEM images indicate that the morphology of PCPy particles is similar to that, which is commonly observed for conjugated polymers obtained by the chemical oxidative polymerization and is commonly produced by the formation of solid-state nuclei, followed by the adsorption of precipitated oligomers.65,66 The well regular shape and morphology are the same in all of samples. The SEM images show aggregates composed of smaller PCPy particles. As can be seen from Fig. 6A, the aggregates of chemically synthesized PCPy particles are of several microns in diameter and consist of smaller particles with a globular morphology and approximately of 200–1000 nm in diameter. While, the PCPy particles synthesized by enzyme catalyzed polymerization (Fig. 6B and C) are formed by globules of smaller diameter (ca. 50–150 nm) compared to those prepared by chemical oxidative polymerization. In addition, the particles synthesized at pH 5.0 were smaller in diameter (Fig. 6B) compared to those prepared at pH 2.0 (Fig. 6C). Similar morphology of the PPy particles synthesized by chemical oxidative and enzyme catalyzed polymerization has been reported by other authors.33,67 The increase of surface area of the PCPy particles synthesized by enzyme catalyzed polymerization technique is a feature attractive to develop biomaterials, because rougher PCPy surfaces have better integration to biological tissues.68
Conclusions and future trends
The PCPy particles were synthesized by the GOx catalyzed and chemical oxidative polymerization technique using H2O2 as an oxidant. H2O2, which was produced during catalytic reaction of the GOx, was able to oxidize the PCA in the initiation step resulting in the formation of chemically active cation radicals of the monomer, which yielded the formation of oligomers and polymers. The PCPy formation rate in polymerization solutions with different pH was investigated and compared with the formation rate of the PCPy synthesized by chemical oxidative polymerization. Optimal pH for the enzymatic polymerization was determined at pH 5.0, while for the chemical at pH 2.0. The GOx catalysed the polymerization reaction and had a positive impact on colloidal stability of formed PCPy particles. The FTIR spectra of PCPy were similar to that of pure PPy. But spectra of the PCPy additionally show infrared absorption peaks corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–H groups of carboxylic acid that were absent in the PPy spectrum, indicating the presence of carboxylic groups in the formed PCPy. Additionally, the presence of carboxylic groups was confirmed by potentiometric back-titration. The SEM study revealed a globular morphology for the PCPy particles prepared both by chemical oxidative or the GOx catalyzed polymerization. The PCPy particles were composed of smaller particles. Each separate chemically synthesized PCPy particle was approximately of 200–1000 nm in diameter and was aggregated into larger cluster of PCPy particles of several microns in diameter. While, the PCPy particles synthesized by enzyme catalyzed polymerization was formed by globules of smaller diameter (ca. 50–150 nm) compared to those prepared by chemical oxidative polymerization.
O and O–H groups of carboxylic acid that were absent in the PPy spectrum, indicating the presence of carboxylic groups in the formed PCPy. Additionally, the presence of carboxylic groups was confirmed by potentiometric back-titration. The SEM study revealed a globular morphology for the PCPy particles prepared both by chemical oxidative or the GOx catalyzed polymerization. The PCPy particles were composed of smaller particles. Each separate chemically synthesized PCPy particle was approximately of 200–1000 nm in diameter and was aggregated into larger cluster of PCPy particles of several microns in diameter. While, the PCPy particles synthesized by enzyme catalyzed polymerization was formed by globules of smaller diameter (ca. 50–150 nm) compared to those prepared by chemical oxidative polymerization.
The advantage of the PCPy particles is that the synthesis of the PCPy particles can be declared as ‘environmentally friendly’ since the only hazardous material H2O2, which is used in chemical oxidative polymerization or produced during the GOx catalyzed reaction, is not stabile and excess of the H2O2 is rapidly converted into water and oxygen. Meanwhile, the synthesized PCPy particles have numerous desirable properties with a high potential for successful applications. The facile preparation of the PCPy particles and the modification of carboxylic groups by various functional groups make these polymeric particles particularly suitable for the covalent attachment of proteins and other biologically active materials as bio-receptors. Due to entrapped GOx the applicability of here reported PCPy particles in glucose biosensor design is foreseen. In addition, PCPy particles can be used in biosensors as relatively stable and porous matrices for enzyme immobilization or for biomedical applications in vivo as drug carrier. In such carriers the drug and/or antibody, which is selective towards target cells, can be electrostatically adsorbed or covalently attached to the particle, what allows to achieve targeted and precisely controlled drug delivery.
Acknowledgements
This research was funded by the European Social Fund under the Global Grant measure.References
- M. Ak, M. S. Ak and L. Toppare, Macromol. Chem. Phys., 2006, 207, 1351 CrossRef CAS.
- J. H. Kim, A. K. Sharma and Y. S. Lee, Mater. Lett., 2006, 60, 1697 CrossRef CAS.
- J. Wang and M. Musameh, Anal. Chim. Acta, 2005, 539, 209 CrossRef.
- A. D. Bendrea, L. Cianga and I. Cianga, J. Biomater. Appl., 2011, 26, 3 CrossRef CAS PubMed.
- H. H. Ciftci, Y. Oztekin, U. Tamer, A. Ramanaviciene and A. Ramanavicius, Colloids Surf., B, 2014, 123, 685 CrossRef CAS PubMed.
- R. H. Baughman, Synth. Met., 1996, 78, 339 CrossRef CAS.
- J. A. Chikar, J. L. Hendricks, S. M. Richardson-Burns, Y. Raphael, B. E. Pfingst and D. C. Martin, Biomaterials, 2012, 33, 1982 CrossRef CAS PubMed.
- D. Svirskis, J. Travas-Sejdic, A. Rodgers and S. Garg, J. Controlled Release, 2010, 146, 6 CrossRef CAS.
- B. Guo, L. Glavas and A. C. Albertsson, Prog. Polym. Sci., 2013, 38, 1263 CrossRef CAS.
- P. Humpolicek, V. Kasparkova, P. Saha and J. Stejskal, Synth. Met., 2012, 162, 722 CrossRef CAS.
- X. D. Wang, X. S. Gu, C. W. Yuan, S. J. Chen, P. Y. Zhang, T. Y. Zhang, J. Yao, F. Chen and G. Chen, J. Biomed. Mater. Res., Part A, 2004, 68, 411 CrossRef PubMed.
- A. Kausaite-Minkstimiene, V. Mazeiko, A. Ramanaviciene and A. Ramanavicius, Colloids Surf., A, 2015, 483, 224 CrossRef CAS.
- H. V. R. Dias, M. Fianchini, R. M. G. Rajapakse and R. L. Elsenbaumer, Polymer, 2006, 47, 7349 CrossRef CAS.
- K. Junker, I. Gitsov, N. Quade and P. Walde, Chem. Pap., 2013, 67, 1028 CAS.
- F. Zou, L. Xue, X. Yu, Y. Li, Y. Zhao, L. Lu, X. Huang and Y. Qu, Colloids Surf., A, 2013, 429, 38 CrossRef CAS.
- R. Bouldin, S. Ravichandran, A. Kokil, R. Garhwal, S. Nagarajan, J. Kumar, F. F. Bruno, L. A. Samuelson and R. Nagarajan, Synth. Met., 2011, 161, 1611 CrossRef CAS.
- R. Cruz-Silva, J. Romero-Garcia, J. L. Angulo-Sanchez, A. Ledezma-Perez, E. Arias-Marin, I. Moggio and E. Flores-Loyola, Eur. Polym. J., 2005, 41, 1129 CrossRef CAS.
- M. Aizawa, L. Wang, H. Shinohara and Y. Ikariyama, J. Biotechnol., 1990, 14, 301 CrossRef CAS.
- G. Shumakovich, V. Kurova, I. Vasileva, D. Pankratov, G. Otrokhov, O. Morozova and A. Yaropolov, J. Mol. Catal. B: Enzym., 2012, 77, 105 CrossRef CAS.
- G. Otrokhov, D. Pankratov, G. Shumakovich, M. Khlupova, Y. Zeifman, I. Vasileva, O. Morozova and A. Yaropolov, Electrochim. Acta, 2014, 123, 151 CrossRef CAS.
- V. Krikstolaityte, J. Kuliesius, A. Ramanaviciene, L. Mikoliunaite, A. Kausaite-Minkstimiene, Y. Oztekin and A. Ramanavicius, Polymer, 2014, 55, 1613 CrossRef CAS.
- A. Kausaite, A. Ramanaviciene and A. Ramanavicius, Polymer, 2009, 50, 1846 CrossRef CAS.
- A. Vaitkuviene, V. Ratautaite, L. Mikoliunaite, V. Kaseta, G. Ramanauskaite, G. Biziuleviciene, A. Ramanaviciene and A. Ramanavicius, Colloids Surf., A, 2014, 442, 152 CrossRef CAS.
- A. Ramanaviciene, A. Kausaite, S. Tautkus and A. Ramanavicius, J. Pharm. Pharmacol., 2007, 59, 311 CrossRef CAS PubMed.
- S. Geetha, C. R. K. Rao, M. Vijayan and D. C. Trivedi, Anal. Chim. Acta, 2006, 568, 119 CrossRef CAS PubMed.
- G. E. Dunn and G. J. Lee, Can. J. Chem., 1971, 49, 1032 CrossRef CAS.
- S. O. C. Mundle, G. Lacrampe-Couloume, B. S. Lollar and R. Kluger, J. Am. Chem. Soc., 2010, 132, 2430 CrossRef CAS PubMed.
- S. O. C. Mundle and R. Kluger, J. Am. Chem. Soc., 2009, 131, 11674 CrossRef CAS PubMed.
- A. A. Vandersteen, S. O. C. Mundle, G. Lacrampe-Couloume, B. S. Lollar and R. Kluger, J. Org. Chem., 2013, 78, 12176 CrossRef CAS PubMed.
- M. Foschini, A. Marletta, R. C. Faria, D. Leonard, F. Bessueille, N. Jaffrezic-Renault and D. Goncalves, Electroanalysis, 2013, 25, 741 CrossRef CAS.
- M. Foschini, H. S. Silva, R. A. Silva, A. Marletta and D. Goncalves, Chem. Phys., 2013, 425, 91 CrossRef CAS.
- R. Bouldin, S. Ravichandran, R. Garhwal, S. Nagarajan, J. Kumar, F. Bruno, L. Samuelson and R. Nagarajan, ACS Polym. Prepr., 2009, 50, 23 Search PubMed.
- R. Cruz-Silva, E. Amaro, A. Escamilla, M. E. Nicho, S. Sepulveda-Guzman, L. Arizmendi, J. Romero-Garcia, F. F. Castillon-Barraza and M. H. Farias, J. Colloid Interface Sci., 2008, 328, 263 CrossRef CAS PubMed.
- R. Cruz-Silva, P. Roman, A. Escamilla and J. Romero-Garcia, ACS Polym. Prepr., 2009, 50, 475 CrossRef CAS.
- K. Junker, G. Zandomeneghi, L. D. Schuler, R. Kissner and P. Walde, Synth. Met., 2015, 200, 123 CrossRef CAS.
- A. Ramanavicius, A. Kausaite, A. Ramanaviciene, J. Acaite and A. Malinauskas, Synth. Met., 2006, 156, 409 CrossRef CAS.
- H. K. Song, G. Tayhas and R. Palmore, J. Phys. Chem. B, 2005, 109, 19278 CrossRef CAS PubMed.
- A. Kausaite-Minkstimiene, V. Mazeiko, A. Ramanaviciene and A. Ramanavicius, Biosens. Bioelectron., 2010, 26, 790 CrossRef CAS PubMed.
- A. Kausaite-Minkstimiene, V. Mazeiko, A. Ramanaviciene and A. Ramanavicius, Sens. Actuators, B, 2011, 158, 278 CrossRef CAS.
- A. Ramanavicius, A. Kausaite and A. Ramanaviciene, Analyst, 2008, 133, 1083 RSC.
- M. Can, H. Ozaslan, O. Isildak, N. O. Pekmez and A. Yildiz, Polymer, 2004, 45, 7011 CrossRef CAS.
- M. C. Henry, C. C. Hsueh, B. P. Timko and M. S. Freunda, J. Electrochem. Soc., 2011, 148, 155 CrossRef.
- A. J. R. Son, H. Lee and B. Moon, Synth. Met., 2007, 157, 597 CrossRef CAS.
- W. Zheng, J. M. Razal, G. M. Spinks, V. T. Truong, P. G. Whitten and G. G. Wallace, Langmuir, 2012, 28, 10891 CrossRef CAS PubMed.
- A. G. MacDiarmid and A. J. Epstein, Synth. Met., 1995, 69, 85 CrossRef CAS.
- M. Choudhary, I. R. Ul, M. J. Witcomb and K. Mallick, Dalton Trans., 2014, 43, 6396 RSC.
- G. Appel, D. Schmeiβer, J. Bauer, M. Bauer, H. J. Egelhaaf and D. Oelkrug, Synth. Met., 1999, 99, 69 CrossRef CAS.
- T. H. Chao and J. March, J. Polym. Sci., Polym. Chem. Ed., 1988, 26, 743 CrossRef CAS.
- M. Y. Arica and G. Bayramoglu, Biochem. Eng. J., 2004, 20, 73 CrossRef CAS.
- W. H. Eisa, M. F. Zayed, Y. K. Abdel-Moneam and A. M. Abou Zeid, Synth. Met., 2014, 195, 23 CrossRef CAS.
- Y. Liao, X. G. Li and R. B. Kaner, ACS Nano, 2010, 4, 5193 CrossRef CAS PubMed.
- Q. Lu, Microchim. Acta, 2010, 168, 205 CrossRef.
- X. Ning, W. Zhong, S. Li and L. Wan, Mater. Lett., 2014, 117, 294 CrossRef CAS.
- H. W. Ryu, Y. S. Kim, J. H. Kim and I. W. Cheong, Polymer, 2014, 55, 806 CrossRef CAS.
- S. J. Hawkins and N. M. Ratcliffe, J. Mater. Chem., 2000, 10, 2057 RSC.
- M. M. Ayad, J. Mater. Sci., 2009, 44, 6392 CrossRef CAS.
- N. V. Blinova, J. Stejskal, M. Trchova, J. Prokes and M. Omastova, Eur. Polym. J., 2007, 43, 2331 CrossRef CAS.
- M. B. Gomez Costa, J. M. Juarez, M. L. Martinez, A. R. Beltramone, J. Cussa and O. A. Anunziata, Mater. Res. Bull., 2013, 48, 661 CrossRef.
- S. Lamprakopoulos, D. Yfantis, A. Yfantis, D. Schmeisser, J. Anastassopoulou and T. Theophanides, Synth. Met., 2004, 144, 229 CrossRef CAS.
- X. G. Li, H. Y. Wang and M. R. Huang, Macromolecules, 2007, 40, 1489 CrossRef CAS.
- C. K. Ong, S. Ray, R. P. Cooney, N. R. Edmonds and A. J. Easteal, J. Appl. Polym. Sci., 2008, 110, 632 CrossRef CAS.
- W. Ozkazanc, S. Zor, H. Ozkazanc and S. Gumus, Polym. Eng. Sci., 2013, 53, 1131 Search PubMed.
- A. T. Dubis, S. J. Grabowski, D. B. Romanowska, T. Misiaszek and J. Leszczynski, J. Phys. Chem. A, 2002, 106, 10613 CrossRef CAS.
- Y. Hoshina and T. Kobayashi, Engineering, 2002, 4, 139 CrossRef.
- S. Benabderrahmane, S. Bousalem, C. Mangeney, A. Azioune, M. J. Vaulay and M. M. Chehimi, Polymer, 2005, 46, 1339 CrossRef CAS.
- C. He, C. Yang and Y. Li, Synth. Met., 2003, 139, 539 CrossRef CAS.
- H. Wang, T. Lin and A. Kaynak, Synth. Met., 2005, 151, 136 CrossRef CAS.
- P. M. George, A. W. Lyckman, D. A. LaVan, A. Hegde, Y. Leung and A. Rupali, Biomaterials, 2005, 26, 3511 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |