Interconnected mesoporous NiO sheets deposited onto TiO2 nanosheet arrays as binder-free anode materials with enhanced performance for lithium ion batteries†
Guojian Li,
Hao Hu,
Qiancheng Zhu and
Ying Yu*
Institute of Nanoscience and Nanotechnology, College of Physical Science and Technology, Central China Normal University, 430079, China. E-mail: yuying01@mail.ccnu.edu.cn; Tel: +86-27-67867037
First published on 9th November 2015
Abstract
To meet the requirement of high-performance lithium ion batteries, transition metal oxides have been taken into considerable account to take the place of the commercialized anode material, graphite, which fails to reach a high capacity and satisfactory cycle life in long-term usage. In this study, TiO2 nanosheet arrays, suffering very little volume changes upon lithium ion intercalation/deintercalation, were synthesized through a facile hydrothermal method as a stable backbone for subsequent chemical bath deposition of interconnected mesoporous NiO sheets with a theoretical capacity of 718 mA h g−1, which is to offset the intrinsically low capacity of TiO2. The specific surface area for the prepared composites increased by 40.4% and the charge transfer resistance descended remarkably compared with that of pure TiO2 nanosheet arrays. The unique TiO2@NiO array structure delivered an average capacity of 420.0 mA h g−1 during 100 cycles at a constant current of 200 mA g−1. The rate performance was improved to be 199.2 mA h g−1 at 1.6 A g−1 and 278.0 mA h g−1 when back to 200 mA g−1, which is better than the pure TiO2 and NiO. A multitude of factors are responsible for the improved performance, including a larger contact surface between the electrode and electrolyte, a shorter Li ion diffusion pathway within the active material, a higher lithium storage capacity of the overall electrode plus ample open space to accommodate volume variation, which all result from the strategy of effective nanoscale structuring and surface modification. The as-designed batteries are free of binders and conductive additives, which is profitable for mass production and convenient for simplifying the manufacturing protocol.
Introduction
Energy storage and conversion devices, such as lithium ion batteries and supercapacitors,1 are extensively applied in ubiquitous electronic products like cell phones, laptops, watches, and so on. Widespread use of lithium ion batteries is attributed to their long life span, high energy density, and high working potential as well as a no-memory effect.2,3 Up to date, for anodic materials, graphite has been regarded as the most sophisticated commercialized anode, whose interlayers are intercalated by lithium ions from the cathode during the charging process. On the contrary, Li ions are released during the discharging process and move backwards to the cathode to produce currents and power the external circuit. Nevertheless, an intrinsically low capacity of graphite constrains its further application in large-scale equipment like hybrid vehicles. Moreover, working under a low potential may cause lithium dendrite deposition on the graphite, which can puncture the separating membrane to induce a short circuit and a battery explosion.4Therefore, in order to ameliorate the performance of the anode for lithium ion batteries, transition metal oxides are under broad investigation to take the place of graphite.5 It is promising to apply them in virtue of their high theoretical capacity, abundance and non-toxicity to the natural environment. Miscellaneous transition metal oxides, like MnO2, SnO2, Fe2O3, Co3O4, etc., have shown good performance when they are used as anode materials.6,7 However, in contrast to graphite, those oxides, including the ones mentioned above, electrochemically react with lithium ions to produce metallic particles and amorphous Li2O, which results in great volume expansion. The volume expansion cracks the electrodes and disconnects the active material from the current collectors, leading to a great irreversible loss of capacity upon cycling and a short life span. In addition, the rate performance is severely hampered by poor kinetics of the transition metal oxides.
Fortunately, amongst a variety of metal oxides, titanium dioxide (TiO2) sheds light on these problems. TiO2 suffers very little volume changes during charging because Li ions intercalate into the lattice of TiO2 to form LixTiO2 instead of the formation of Li2O. A relatively high working potential plateau of 1.5–1.7 V (vs. Li/Li+) avoids the formation of lithium dendrites and passivates the solid electrolyte interface (SEI), which enables Li ions to penetrate to react with the active material.8 Notwithstanding, TiO2 is in possession of a low capacity of 335 mA h g−1 and a poor ionic and electronic conductivity. Therefore, resolutions to these intractable problems are highly desired. On one hand, nanoscale array architectures not only increase the electrochemical reaction surface area but also effectively prevent agglomeration of the active materials, fostering adequate surface exposure and efficient charge transfer. Furthermore, the nanoscale architectures can shorten the Li ion diffusion pathway so as to improve the reaction kinetics.9 A multitude of morphologies, such as TiO2 nanotubes,10 nanospheres,11 nanofibers12 and nanoflakes, are successfully synthesized to show excellent performance. In particular, TiO2 with {001} high energy facets is favorable for lithium storage.13 On the other hand, to compensate for the low capacity of TiO2, the alternative is to coat another metal oxide of high capacity, like NiO14 (718 mA h g−1, nearly two times the capacity of graphite), onto the TiO2 structure.15 TiO2 is a type of material that is compatible with other oxides.16 NiO stands out from other alternatives because of its non-toxicity and lower cost, compared to that of Co-based oxides.17 Several studies have shown that a NiO nanostructure is facile to prepare via solution-based synthesis and behaves well in collaboration with other oxides as an anode. Xiong18 et al. synthesized Fe2O3@NiO core/shell nanorod arrays on carbon cloth as an anode with an enhanced capacity of the Fe2O3 electrode by about 40%. NiO@TiO2 nanopowders reported by Choi19 et al. also had a significantly increased capacity compared with TiO2. Even pure NiO in different morphologies like yolk–shell and cubic NiO nanopowders,20 NiO octahedra,21 ultrathin NiO nanosheets22 and its incorporation with graphene23,24 can reach a satisfactory electrochemical performance as well, implying that NiO is a prominent candidate for anode materials.
Inspired by the previous studies, herein, hierarchical TiO2@NiO core/shell nanosheet arrays are proposed as an anode material for lithium ion batteries. It is feasible to fabricate the TiO2@NiO core/shell nanosheet arrays through a hydrothermal method25 followed by chemical bath deposition based on our recent report.26 TiO2 sheet arrays directly grown on a Ti substrate served as a stable backbone for the subsequent deposition of interconnected NiO sheets, which contributed to the majority of the capacity. The TiO2@NiO core/shell nanosheet arrays designed in this study have several merits as illustrated in Fig. 1. Firstly, the TiO2 sheets can not only serve as an electrochemically active and stable backbone, but they also function as an intermediate for collecting electrons and directionally transporting them to the Ti substrate. Secondly, the unique sheet-on-sheet structure at the nanoscale with a large surface area, shorter diffusion path and open space between the sheets may be beneficial to accommodate the volume expansion and fully contact with the electrolyte to enable more Li+ diffusion for a fast conversion reaction, which is crucial to the improvement of the cycle life and rate performance. Last but not least, due to the zero-strain property of TiO2, the whole array structure can attach to the current collector steadily to ensure good electrical contact.27 To the best of our knowledge, this “NiO sheets on TiO2 sheets” structure has never been reported yet. The theoretical capacity of our prepared composite electrode was calculated to be 526.5 mA h g−1, while in the charge/discharge process at a constant current of 200 mA g−1, the electrode still had an average capacity of 420.0 mA h g−1 within 100 cycles, with only 179.8 mA h g−1 and 281.6 mA h g−1 for the control TiO2 and NiO, respectively. Although the initial capacity loss was unsatisfactory, the coulombic efficiency recovered to be around 99% just after a few cycles. The rate performance has been enhanced in comparison with either singular TiO2 or NiO electrodes. Finally, it is worth noting that our electrode is convenient to fabricate and suitable for mass production in industry.
 | ||
| Fig. 1 Schematic illustration for the merits of the as-designed hierarchical TiO2@NiO nanosheet arrays. | ||
Experimental
Synthetic procedures
A TiO2 nanosheet array backbone was synthesized through a facile hydrothermal method based on our previous report.26 Prior to synthesis, a Ti foil was polished and ultrasonicated in deionized water, acetone and ethanol for 10 minutes, respectively. Then, the Ti foil was placed in a Teflon-lined stainless steel autoclave loaded with 40 mL 1 M NaOH solution. The autoclave was sealed and kept in an electronic oven heating at 180 °C for 14 hours. After that, the foil was taken out and rinsed with deionized water followed by immersion into 1 M HCl solution for 40 minutes. Lastly, the foil was annealed at 500 °C for 1 hour in ambient atmosphere.NiO was deposited onto TiO2 to form TiO2@NiO nanosheet arrays through a chemical bath deposition method.28 Typically, 20 mL aqueous ammonia (25–28%) was added into a mixture of 100 mL 1 M NiSO4·4H2O and 80 mL 0.25 M K2S2O8. Thereafter, the as-synthesized TiO2 substrate was placed vertically in the solution for 10 minutes at room temperature. Finally, the substrate was taken out and rinsed with deionized water and then annealed at 350 °C for 2 hours in argon. It was easy to vary the amount of NiO by controlling the reaction time.29 The synthesis process is illustrated in Fig. 2, and the prepared arrays can be employed directly as an anodic electrode in the absence of any binders or additives.
In order to compare the performance of the control electrode, pure TiO2 and NiO nanosheets were synthesized under the same conditions as described above. The pure NiO sheets were deposited on Ti foil instead. Time-controlled experiments to chemical bath deposition of NiO were carried out to investigate the mechanism. All reagents mentioned in this work were purchased from Sinopharm Chemical Reagent Co., Ltd (China). All reagents are used directly without further purification.
Structural characterization, battery assembly and electrochemical measurement
X-ray diffraction (XRD, X’PertPRO MRD, PANalytical, Netherlands, Cu Kα radiation) was used to determine the phases of the samples. Their morphology was observed by field emission scanning electron microscopy (FESEM, JEOL JSM-6700F) equipped with energy dispersive spectroscopy (EDS, EDAX genesis 7000), and transmission electron microscopy (TEM, JEM-2010HT) including high-resolution transmission electron microscopy (HRTEM, JEM-2010 FEF). The Brunauer–Emmett–Teller (BET) method helped to calculate the specific surface area with a BELSORP-mini (BEL) instrument. The Barrett–Joyner–Halenda (BJH) model was exploited to evaluate the pore distribution. Raman spectra (LabRAM HR JY-Evolution, excitation wavelength λ = 532 nm) were collected to analyse the structure information of the samples.Battery performance was measured with a CR2016 half coin cells system. The as-prepared sample was employed as a working electrode with the metallic Ti substrate as the current collector, while a slice of lithium foil was used as the counter and reference electrodes. The electrolyte was composed of LiPF6 (1 M) dissolved in a mixture of ethylene carbonate (EC), ethylmethyl carbonate (EMC) and dimethyl carbonate (DMC) with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Polypropylene films (Celgard2000) were used as the separator. Assembly of the batteries was conducted in a glove box (Mbraun) with a flow of inert argon. The charge/discharge measurement to the cells under various current densities was performed on a BTS-55 Neware battery testing system (Shenzhen, China) at room temperature. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured with electrochemical working stations (CHI660E and PARSTAT 2273) within 0.05–3.0 V and 10 mHz to 100 kHz respectively. Each voltage mentioned in this paper was versus Li/Li+. The weight determination of active materials is presented in the ESI.†
1. Polypropylene films (Celgard2000) were used as the separator. Assembly of the batteries was conducted in a glove box (Mbraun) with a flow of inert argon. The charge/discharge measurement to the cells under various current densities was performed on a BTS-55 Neware battery testing system (Shenzhen, China) at room temperature. Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were measured with electrochemical working stations (CHI660E and PARSTAT 2273) within 0.05–3.0 V and 10 mHz to 100 kHz respectively. Each voltage mentioned in this paper was versus Li/Li+. The weight determination of active materials is presented in the ESI.†
Results and discussion
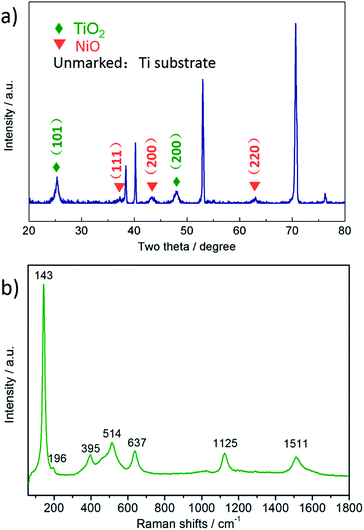
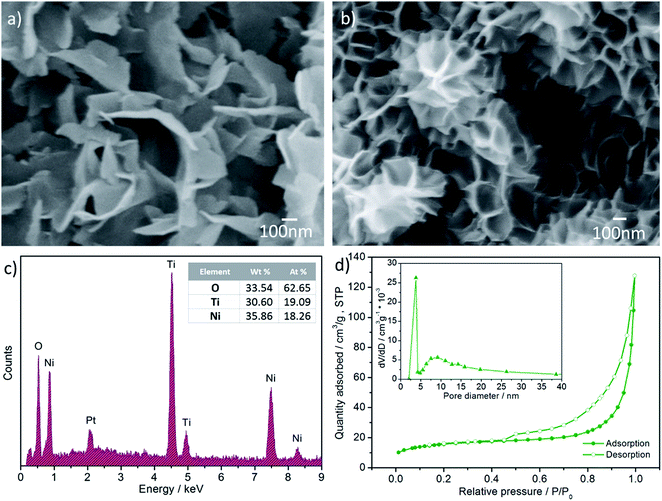
The XRD pattern of the as-prepared composite is shown in Fig. 3(a). All peaks from the composite agreed well with anatase TiO2 (JCPDS no. 1-562), NiO (JCPDS no. 47-1049) and Ti (JCPDS no. 1-1197), indicative of the successful synthesis of TiO2@NiO nanosheets on a Ti substrate. Two obvious peaks located at around 25.3° and 48.0° were assigned to the (101) and (200) planes of anatase TiO2, which showed a supreme performance among a number of TiO2 polymorphs.30 Two broad peaks at 37.2° and 43.2° corresponding to the (111) and (200) planes of NiO indicated the lower crystallinity. Raman analysis is utilized to further confirm the phases of the as-prepared sample. As shown in Fig. 3(b), a Raman characteristic spectrum of anatase TiO2 is found with peaks at 143, 196, 395, 514 and 637 cm−1, which correspond to the Eg, Eg, B1g, A1g and Eg vibration modes, respectively.31,32 Two remaining peaks located at 1125 and 1511 cm−1 are in accordance with two longitudinal optical phonon (2LO) and two-magnon (2M) scattering modes of NiO,9,33 respectively, which indicates that NiO was successfully deposited onto the TiO2 surface.SEM images display the morphologies of the pristine TiO2 nanosheet arrays and the hierarchical TiO2@NiO nanosheet arrays. In Fig. 4(a), ahead of the NiO deposition, there are a number of nanoscale sheets distributed evenly on the Ti substrate with the thicknesses ranging from 5 to 25 nm. These thin TiO2 sheets with a short diffusion pathway served as a stable backbone to support NiO of a high capacity, guaranteeing stability during the charge/discharge process. After precipitation, NiO sheets were also homogeneously attached to the surface of the TiO2 sheets for the formation of a unique sheet-on-sheet structure, as shown in Fig. 4(b). The NiO sheets, in the form of an interconnected network with an average thickness of about 6 nm, shared a similar morphology with the TiO2 backbone. More importantly, the NiO sheets diverged in different directions instead of agglomerating to form a flower-like morphology. This open structure can be beneficial for active reaction sites to be fully exposed. Besides, energy dispersive X-ray spectroscopy (EDS) was employed to measure the elemental ratio of the hybrid. EDS analysis (Fig. 4(c)) shows that the atom percentage of the elements O, Ti and Ni was 62.65%, 19.09% and 18.26%, respectively. As a result, the stoichiometric number ratio was approximated as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, suggesting that TiO2 coated with NiO in an equal molar value. Based on this analysis, the gravimetric capacity for the as-prepared sample was calculated easily by the following equation: 335 mA h g−1 × 50% + 718 mA h g−1 × 50% = 526.5 mA h g−1. The Pt signal resulted from Pt sputtering before measurement in order to increase the electronic conductivity. In addition, it is noted that the active material was scraped off from the Ti substrate to avoid miscalculation. As displayed in the SEM images, the overall structure was composed of tiny nanoscale sheets with a giant exposed surface, and there was large space in between sheets, which may allow prompt permeation of Li ions from the electrolyte into the active material and facilitate volume expansion/contraction during cycling.34 Through the strategy of additional NiO sheet deposition onto the backbone, it was not surprising to observe an augmented surface area via intuitive SEM images. To strictly verify that the surface area did indeed increase, the Brunauer–Emmett–Teller (BET) method was applied to examine the TiO2@NiO hybrid as well as the TiO2 backbone. Nitrogen adsorption and desorption isotherms are presented in Fig. 4(d). The adsorption and desorption isotherms exhibited a type IV shape along with a type H3 hysteresis loop according to the classification by IUPAC.35 The BET specific surface area for the hybrid sample was 55.69 m2 g−1 while only 39.66 m2 g−1 was for the TiO2 backbone (Fig. S1 in the ESI†), increasing by about 40% for the composite compared with pure TiO2 sheets. This increased surface area can enable more Li ion diffusion, resulting in an improved power density and rate performance. It is noteworthy that the type IV isotherms were associated with capillary condensation taking place in mesopores, demonstrating the existence of a mesoporous structure for the as-prepared material. The pore distribution was derived from the desorption data with the help of the Barrett–Joyner–Halenda (BJH) model and the average pore size was calculated to be 7.65 nm. Such a mesoporous structure played an important role in facilitating electrolyte infiltration and volume variation upon cycling.
1, suggesting that TiO2 coated with NiO in an equal molar value. Based on this analysis, the gravimetric capacity for the as-prepared sample was calculated easily by the following equation: 335 mA h g−1 × 50% + 718 mA h g−1 × 50% = 526.5 mA h g−1. The Pt signal resulted from Pt sputtering before measurement in order to increase the electronic conductivity. In addition, it is noted that the active material was scraped off from the Ti substrate to avoid miscalculation. As displayed in the SEM images, the overall structure was composed of tiny nanoscale sheets with a giant exposed surface, and there was large space in between sheets, which may allow prompt permeation of Li ions from the electrolyte into the active material and facilitate volume expansion/contraction during cycling.34 Through the strategy of additional NiO sheet deposition onto the backbone, it was not surprising to observe an augmented surface area via intuitive SEM images. To strictly verify that the surface area did indeed increase, the Brunauer–Emmett–Teller (BET) method was applied to examine the TiO2@NiO hybrid as well as the TiO2 backbone. Nitrogen adsorption and desorption isotherms are presented in Fig. 4(d). The adsorption and desorption isotherms exhibited a type IV shape along with a type H3 hysteresis loop according to the classification by IUPAC.35 The BET specific surface area for the hybrid sample was 55.69 m2 g−1 while only 39.66 m2 g−1 was for the TiO2 backbone (Fig. S1 in the ESI†), increasing by about 40% for the composite compared with pure TiO2 sheets. This increased surface area can enable more Li ion diffusion, resulting in an improved power density and rate performance. It is noteworthy that the type IV isotherms were associated with capillary condensation taking place in mesopores, demonstrating the existence of a mesoporous structure for the as-prepared material. The pore distribution was derived from the desorption data with the help of the Barrett–Joyner–Halenda (BJH) model and the average pore size was calculated to be 7.65 nm. Such a mesoporous structure played an important role in facilitating electrolyte infiltration and volume variation upon cycling.
Moreover, with the purpose of understanding the growth mechanism of NiO, chronological SEM images are provided in Fig. S2 (ESI).† The formation of the NiO sheets was related to the evolution of NiOOH sheets, which mainly involved the two processes of self-assembly and oriented attachment, aside from annealing treatment.28,36 At the initial stage of the deposition, tremendous Ni-based hydroxide mesocrystals were nucleated by self-assembling in the super-saturated solution. Then they preferentially attached to the TiO2 backbone to form active nucleation centers so that the overall surface energy could be diminished. As the deposition started, these centers played important roles in guiding the succeeding direction-oriented growth of the planar structure, and eventually the structure of the precursor NiOOH sheets deposited on the TiO2 sheets was obtained. Then after annealing treatment, the NiOOH sheets in situ dehydrated to NiO sheets. Due to the assembly and attachment processes, numerous mesopores inside the NiO sheets originating from interstitial voids between nanocrystallites were formed.37 The cross-sectional SEM images of the TiO2 nanosheet arrays and TiO2@NiO hybrid nanosheet arrays are also provided in Fig. S3 (ESI†) to verify the formation of the special morphology.
TEM images show more detailed information on the morphological and structural features of the composite. The TiO2 scaffold was uniformly covered with many thin NiO sheets, which robustly attached to the scaffold even after sonication prior to the TEM measurement. This suggested good stability and mechanical attachment between the two phases (Fig. 5(a)). As the image shows (inset in Fig. 5(a)), the NiO sheets were approximately 100 nm in height relative to the surface of the TiO2. They exhibit a transparent character in the image, indicating their extremely thin thickness, which may remarkably reduce the Li ion diffusion pathways. To delve deeper into the lattice structure, high-resolution TEM (HRTEM) was utilized. Distinctive lattice fringes were found in both the TiO2 backbone and NiO sheets as displayed in Fig. 5(b). The lattice fringe with an interplanar spacing of 0.33 nm was assigned to the (101) plane of anatase TiO2. Additionally, the lattice fringes with a d-spacing of 0.25 nm and 0.21 nm corresponded to the planes (111) and (200) of the cubic NiO phase respectively, in good agreement with the XRD pattern. For more explicit fringes, a selected area is magnified in the inset of Fig. 5(b). A closer look at the exterior NiO sheets (Fig. 5(c)) reveals that those sheets were constituted of substantial tiny NiO particles with a small size of approximate 2.5 nm, from which a porous structure was consequently formed. It coincides with the result from the BET measurement above as well. Thus it can be further confirmed that the precursor NiOOH for the NiO sheets was assembled by tiny particles nucleated in the super-saturated solution, gradually attaching together to form a directional planar structure. The selected area electron diffraction (SAED) pattern (Fig. 5(d)) shows a series of bright concentric rings, which represents the polycrystalline nature of the material. With careful calculation, the diffraction rings were determined to originate from the planes (101) and (200) of anatase TiO2 as well as the planes (111), (200) and (220) of NiO, precisely consistent with the results from the XRD pattern.
The unique structure was employed as an anode material for lithium ion batteries. Cyclic voltammetry (CV) for the first three cycles was performed to give an insight into the electrochemical reaction during the charge/discharge process. The CV measurement was conducted at a sweep rate of 0.3 mV s−1 in the voltage range from 0.05 to 3 V. In Fig. 6(a), in the first cycle, two reduction peaks are observed during the anodic process, in which the lithium ions insert into the anode. The reduction peak at 0.3 V was attributed to the Li ion initial reaction with NiO to form metallic nickel particles and the amorphous Li2O concomitant with the formation of a complicated SEI.38 It is already reported that metallic nickel particles derived from NiO reduction have positive impacts. For instance, Ni nanoparticles increased the electrical conductivity of the less conductive metal oxide electrode and they fostered the decomposition of Li2O to enhance the specific capacity upon charging.39 The other broad peak located at around 1.1 V was associated with the insertion of Li ions into the lattice of TiO2, which is somehow restrained in the first cycle. Apart from the reduction peaks, there are two oxidation peaks at 1.68 V and 2.36 V in accordance with partial decomposition of the SEI, nickel particle oxidation to NiO, and Li ion extraction from LixTiO2 to form TiO2, where lithium ion diffusion was dominant.40–42 In subsequent cycles, the reduction peaks shifted to 0.59 V and 1.59 V due to a few reasons including structural modifications and the polarization effect, as well as possible formation of defective NiOδ, a new phase, during the first oxidation in a different reaction pathway reported recently,43–45 whereas the oxidation peaks almost remained the same with only a slight shift. Moreover, one could perceive that the close area between the curves shrank, implying an irreversible capacity loss during cycling. However, there was no significant area change between the second and third cycle, meaning that good stability was obtained soon. For reference, the electrochemical reactions concerned during cycling are listed below:
| TiO2 + xLi+ + xe− ↔ LixTiO2 (0 ≤ x ≤ 1) |
| NiO + 2Li+ + 2e− ↔ Li2O + Ni |
The cycle performance was measured by a galvanostatic charge/discharge method at a current density of 200 mA g−1. Fig. 6(b) shows the cycle performance of the TiO2@NiO electrode accompanied with the pure TiO2 and NiO electrode for comparison. It is obvious to find that the TiO2@NiO electrode transcended the other electrodes in the aspect of discharge capacity despite the first several cycles. Due to a low coulombic efficiency of only 60% at the beginning, the discharge capacity of the TiO2@NiO electrode plummeted from 947.7 mA h g−1 to 541.2 mA h g−1. However, the efficiency climbed up to around 87% soon after the first cycle and stabilized at around 99%. The capacity tended to vary slowly, suggesting that good stability was obtained shortly. The low coulombic efficiency in the beginning was attributed to the partial exfoliation of the active materials derived from volume expansion/contraction and the continuous SEI formation. Actually, incomplete delithiation from the active materials should also be blamed.46 The flat line-shaped cycle performance of TiO2, maintaining an average capacity of 180 mA h g−1 without distinct decrement all the way, indicated its excellent stability, which was also shown in the composite electrode. After 100 cycles, the composite TiO2@NiO electrode still had a capacity of 376.5 mA h g−1, 71.5% of the theoretical capacity, in contrast with only 179.0 mA h g−1 for TiO2 and 176.8 mA h g−1 for NiO. Taking the whole charge/discharge process into consideration, the hybrid TiO2@NiO electrode delivered a capacity of 420.0 mA h g−1 on average at a current density of 200 mA g−1, while only 179.8 mA h g−1 and 281.6 mA h g−1 was obtained for TiO2 and NiO, respectively. Apparently, the capacity for NiO decreased rapidly without the support of a stable backbone. From this point of view, TiO2 arrays provided a solid foundation for a decent cycling performance. In general, the composite electrode was superior to both the NiO electrode and TiO2 electrode with respect to capacity and stability, indicative of the rationality of our design. The delicate incorporation of both materials with the respective advantages compensated the drawback of each other, resulting in an improvement of the overall electrode. What deserves to be mentioned is that the practical capacity was higher than that from theory. This phenomenon appears to be common in many transition metal oxide electrodes. There is a possibility that the additional capacity mainly originated from the reversible reaction of LiOH, a byproduct of the conversion reaction, to form LiH and the SEI.47 Additionally, an interfacial charge storage mechanism was also believed to account for the extra capacity.48 The galvanostatic charge/discharge curves are presented to show the charge or discharge process in Fig. 6(c). During the first discharge process, a long slope ranging from 2.0 to about 0.75 V followed by a small plateau around 0.6 V is observed, corresponding to the lithiation process. There is also a small plateau at about 2.2 V in the charge process because of the existence of TiO2 in good agreement with the CV profile above. Evidently, the 100th charge/discharge curves are quite different from the first cycle for the changes of the electrode and the irreversible capacity loss.
The rate performance was tested at a range of current densities and is presented in Fig. 6(d). With the increase of current density, the capacity decreased to different extents for all three electrodes. As expected, the stable TiO2 backbone behaved extraordinarily under different current densities from 200 mA g−1 to 1.6 A g−1 despite its intrinsically low capacity. Even after charge/discharge at high current densities, its capacity was restored to 162 mA h g−1, only 14% lower than the initial 188.7 mA h g−1, when it was switched back to a low current density of 200 mA g−1. With regard to the TiO2@NiO electrode, its capacity on average decreased significantly from 700.5 mA h g−1 at 200 mA g−1 to 199.2 mA h g−1 at 1.6 A g−1 and was restored to 278.0 mA h g−1 at 200 mA g−1 again, indicating the occurrence of structural damages to the electrode at high current densities. It can be ascribed to the poor kinetics and pulverization of NiO.49 In contrast with pure TiO2 and NiO, the rate capacity transcended that of either individual component, which stemmed from the hierarchical structure with an increased exposed active surface for more Li ions to diffuse concurrently into the material. Furthermore, a well-defined thickness of the nanosheets shortened the diffusion time (t) by decreasing the path length (L) according to the equation  , in which the coefficient D is a constant for a particular material.50 Since more Li ions diffused into and reacted with the active material in a short period, the electrode was charged/discharged at high rates. In addition, the charge/discharge curves in Fig. S4 (ESI†) were different since the plateaus shifted to a higher potential in the charge cycle as the current increased because of the kinetic effects of the material.51
, in which the coefficient D is a constant for a particular material.50 Since more Li ions diffused into and reacted with the active material in a short period, the electrode was charged/discharged at high rates. In addition, the charge/discharge curves in Fig. S4 (ESI†) were different since the plateaus shifted to a higher potential in the charge cycle as the current increased because of the kinetic effects of the material.51
Electrochemical impedance spectroscopy (EIS) was performed to elucidate the advantages of the TiO2@NiO over the pure TiO2 and NiO electrode. Three electrodes were measured at a fully charged state after the fourth cycle ranging from 100 kHz to 10 mHz with a voltage amplitude of 5 mV. Nyquist plots of all three electrodes are shown in Fig. 7. They share a similar shape, all consisting of a depressed semicircle in the high-frequency region and a subsequent slope in the low-frequency region. Amongst the three electrodes, TiO2@NiO possessed the smallest diameter of the semicircle with 85.2 Ω, which refers to the lowest charge transfer resistance Rct at the interface between the electrolyte and electrode, and the most rapid electrochemical reaction, that was advantageous to the rate performance. The reason why TiO2@NiO had a smaller resistance than that of TiO2 (208.3 Ω) and NiO (240.6 Ω) may be attributed to the synergistic effect induced by the deft combination of the TiO2 and NiO.52 In the case of the combination of the TiO2 and NiO here, thin NiO sheets attached tightly to the surface of the TiO2 backbone, ensuring efficient Li+ and charge supply from each other. On the other hand, an appropriate amount of NiO sheets with a large exposed surface and open structure was conducive to full electrolyte/electrode contact and a swift electrochemical reaction. Besides, the tiny thickness of the NiO sheets helped to shorten the diffusion pathway in addition to reducing the activation energy, also resulting in rapid charge transfer.53,54 All the aspects above regarding the configuration of the TiO2@NiO hybrid structure synergistically contribute to the improved kinetics. This means that active surface modification by coating metal oxides had a positive impact on the interfacial charge transfer. The inset in Fig. 7 is an equivalent circuit for the hybrid electrode. R0 and RSEI stand for the resistance of the active material itself and the SEI film respectively. W refers to the Warburg diffusion impedance. Cdl is the double-layer capacitance and CSEI is the capacitance associated with RSEI.
 | ||
| Fig. 7 Nyquist plots of the three electrodes in the scanning range from 100 kHz to 10 mHz. Inset is the equivalent circuit for the hybrid TiO2@NiO electrode. | ||
Beyond question, the improved electrochemical performance was obtained after coating the NiO sheets onto the surface of the TiO2 backbone. To have an in-depth understanding of the mechanism, the aforementioned time-controlled experiments were carried out. The chemical bath deposition durations were chosen to be 8, 10 and 12 minutes for comparison. Given the chronological SEM images prior to cycling in Fig. S2 (ESI),† it was a rapid deposition process.29 With the deposition going on (Fig. 8), the NiO sheets were grown homogeneously on the surface of the TiO2 backbone to form an interconnected network, from an inadequate to a moderate amount of NiO, then finally to an overloaded stage. It is predicted that, upon cycling, insufficient NiO failed to increase the capacity of the overall electrode since the TiO2 limitedly contributed to the capacity, while excessive NiO sheets diminished the neighbouring space which buffered the volume expansion, resulting in mutual extrusion to break down the structure of the electrode and the final poor cycle performance. Therefore, we propose that a moderate amount of NiO sheets was supposed to achieve the best performance. To prove our hypothesis, the galvanostatic charge/discharge measurement was conducted again at a current density of 200 mA g−1 to determine the capacity performance. The discharge capacity of NiO sheets deposited for different durations is shown in Fig. S5 (ESI).† It reveals that in our case, ten minute deposition was optimal as after 15 times of cycling, its capacity transcended the other two and its excellent stability remained. The excessive amount of NiO (12 minute deposition in our case) delivered a considerable capacity at first but then decayed quickly due to the structure damage mentioned above. That is the reason why the case of ten minute deposition is selected as a proof of concept and discussed in detail within this paper. An optimal deposition amount is necessary for unleashing the performance of the materials.
In order to further confirm our hypothesis, SEM images after 15 times of cycling at 200 mA g−1 were taken for more detailed morphological information (Fig. 9). It is distinguishable that in the case of an excessive NiO amount, those sheets covering the TiO2 became thicker and denser. Before cycling (Fig. S2 in ESI†), the NiO sheets were thin and stood independently with each other while after cycling they swelled, squeezed and aggregated into stacks, which was likely to block subsequent lithium ion migration and likewise reduce the exposed active surface. The space in between each sheet decreased dramatically because of the volume expansion and the stacking inducing extrusion was harmful for the electrode structure. On the other hand, an opposite situation took place in terms of the case of an insufficient amount. The outline of the substrate TiO2 and the trace of the NiO were clearly seen. Although the outline was maintained, they failed to deliver a high gravimetric capacity on account of being subject to little active material. As for a moderate amount of NiO deposition, the contour of the primary structure was still observed even with volume variation. Many NiO sheets stood free as ever and there was no evident stack of NiO sheets in the image. Therefore, a reasonable capacity and a stable cycling performance were achieved simultaneously. Moreover, EIS data unfolded in-depth electrochemical differences induced by the deposition amount. As illustrated in Fig. S6 (ESI),† the 10 min electrode had the smallest transfer resistance among all conditions. These results corroborate that an excess amount of NiO was not necessarily beneficial for the electrochemical performance. Excessive deposition led to structural deterioration and interface formation with high potential barriers which impeded subsequent electron transfer.55 It straightforwardly follows that the precipitation amount of the exterior NiO played an important role in the cycling performance. In this regard, active materials are not generally deposited as much as possible but are deposited reasonably to achieve a balance between stability and capacity, so as to optimize the electrochemical performance as a whole. To sum up, these SEM images and EIS data are in agreement with the result from the comparative study of the capacity in Fig. S4† and match our prediction, which is solid evidence to validate our aforementioned hypothesis.
 | ||
| Fig. 9 SEM images of electrodes after fifteen times of cycling (the arrows indicate the spots where aggregation occurs). | ||
In brief, by the combination of the merits of TiO2 with extraordinary stability and NiO with high capacity, the as-prepared hierarchical TiO2@NiO electrode exhibited improved cyclability and rate performance as compared to the pure TiO2 and NiO electrode.56,57 In contrast with the TiO2/NiO nanopowders reported,19 the surface area of the prepared sample in this work increased profoundly, over twice that of the nanopowders, on account of the hierarchical array morphology. Moreover, as far as we are concerned, there are current literatures with regard to other forms of TiO2 arrays decorated with NiO, which are incapable of enduring up to 100 times of cycling.42,58,59 Whereas this work accomplished it with a long life span and decent capacity. In other words, it is the first time for hybrid TiO2@NiO arrays to obtain such a gratifying performance. Assuredly, multiple factors are responsible for this impressive improvement, including a larger contact surface between the electrode and the electrolyte, shorter Li ion diffusion pathways within the active material, a higher lithium storage capacity of the overall electrode plus ample open space to accommodate volume variation, which are all achieved by effective nanoscale structuring and a surface modification strategy to form a unique hierarchical structure.60 Furthermore, the as-assembled cells are free of binders and conductive additives, which not only makes more room for electrochemically active material in batteries of an identical size but also spares reagents and a prime cost for profitable and scalable production in industry.
Conclusions
A hierarchical TiO2@NiO nanosheet array was synthesized through a facile hydrothermal method in combination with a mild chemical bath deposition procedure. When applied as an anode in lithium ion batteries, this hybrid electrode exhibited an average discharge capacity of 420.0 mA h g−1 during 100 cycles with nearly 100% coulombic efficiency at a constant current density of 200 mA g−1. Even after 100 cycles, it still rendered a capacity of 376.5 mA h g−1, which is close to but higher than graphite’s theoretical capacity. In addition, the electrode of the hierarchical TiO2@NiO nanosheet array had a much improved rate performance compared to the worse performance of pure NiO and TiO2. The much better electrochemical properties were credited to the effective NiO deposition and unique morphology. Besides, the deposition amount of mesoporous NiO sheets was critical for the cycling performance. In summary, our unique design of the nanoscale sheet-on-sheet array, which avoids particle agglomeration, is very beneficial to the increased active surface and shorter diffusion time, which outperforms those of the conventional bulk material or powder-assembled electrodes. The simple design of a hierarchical composite electrode in this case is likely to be extended to other metal oxide materials for energy storage devices with supreme electrochemical performance.Acknowledgements
This work was financially supported by National Science Foundation of China (no. 21377044 and 21573085), the Key Project of Natural Science Foundation of Hubei Province (no. 2015CFA037), Wuhan Planning Project of Science and Technology (no. 2014010101010023) and self-determined research funds of CCNU from the colleges’ basic research and operation of MOE (no. CCNU15ZD007 and CCNU15KFY005).References
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167 CrossRef CAS PubMed.
- N. S. Choi, Z. H. Chen, S. A. Freunberger, X. L. Ji, Y. K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. H. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994 CrossRef CAS PubMed.
- P. Poizot, S. Laruelle, S. Grugeon, L. Dupont and J. M. Tarascon, Nature, 2000, 407, 496 CrossRef CAS PubMed.
- B. L. Ellis, P. Knauth and T. Djenizian, Adv. Mater., 2014, 26, 3368 CrossRef CAS PubMed.
- H. Hu, H. Y. Cheng, G. J. Li, J. P. Liu and Y. Yu, J. Mater. Chem. A, 2015, 3, 2748 CAS.
- L. Xin, Y. Liu, B. J. Li, X. Zhou, H. Shen, W. X. Zhao and C. L. Liang, Sci. Rep., 2014, 4, 4479 Search PubMed.
- A. S. Aricò, P. Bruce, B. Scrosati, J. M. Tarascon and W. V. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed.
- P. Roy, S. Berger and P. Schmuki, Angew. Chem., Int. Ed., 2011, 50, 2904 CrossRef CAS PubMed.
- L. Gao, X. R. Li, H. Hu, G. J. Li, H. W. Liu and Y. Yu, Electrochim. Acta, 2014, 2, 231 CrossRef.
- S. H. Nam, H. S. Shim, Y. S. Kim, M. A. Dar, J. G. Kim and W. B. Kim, ACS Appl. Mater. Interfaces, 2010, 2, 2046 CAS.
- X. D. Wang, Z. D. Li, J. Shi and Y. H. Yu, Chem. Rev., 2014, 114, 9346 CrossRef CAS PubMed.
- D. Su, M. Ford and G. X. Wang, Sci. Rep., 2012, 2, 924 Search PubMed.
- Q. C. Zhu, H. Hu, G. J. Li, C. B. Zhu and Y. Yu, Electrochim. Acta, 2015, 156, 252 CrossRef CAS.
- H. G. Wang, D. L. Ma, X. L. Huang, Y. Huang and X. B. Zhang, Sci. Rep., 2012, 2, 701 Search PubMed.
- A. Caballero, L. Hernán and J. Morales, Energy Fuels, 2013, 27, 5545 CrossRef CAS.
- Q. Q. Xiong, J. P. Tu, X. H. Xia, X. Y. Zhao, C. D. Gua and X. L. Wang, Nanoscale, 2013, 5, 7906 RSC.
- S. H. Choi, J. H. Lee and Y. C. Kang, Nanoscale, 2013, 5, 12645 RSC.
- S. H. Choi and Y. C. Kang, ACS Appl. Mater. Interfaces, 2014, 6, 2312 CAS.
- Z. K. Yang, L. X. Song, R. R. Xu, Y. Teng, J. Xia, L. Zhao and Q. S. Wang, CrystEngComm, 2014, 16, 9083 RSC.
- S. Q. Ci, J. P. Zou, G. S. Zeng, Q. Peng, S. L. Luo and Z. H. Wen, RSC Adv., 2012, 2, 5185 RSC.
- L. Q. Tao, J. T. Zai, K. X. Wang, Y. H. Wan, H.J. Zhang, C. Yu, Y. L. Xiao and X. F. Qian, RSC Adv., 2012, 2, 3410 RSC.
- G. M. Zhou, D. W. Wang, L. C. Yin, N. Li, F. Li and H. M. Cheng, ACS Nano, 2012, 6, 3214 CrossRef CAS PubMed.
- C. H. Wang, X. T. Zhang, Y. L. Zhang, Y. Jia, J. K. Yang, P. P. Sun and Y. C. Liu, J. Phys. Chem. C, 2011, 115, 22276 CAS.
- L. Gao, H. Hu, G. J. Li, Q. C. Zhu and Y. Yu, Nanoscale, 2014, 6, 6463 RSC.
- J. B. Wu, R. Q. Guo, X. H. Huang and Y. Lin, J. Power Sources, 2014, 248, 115 CrossRef CAS.
- X. H. Xia, J. P. Tu, Y. Q. Zhang, X. L. Wang, C. D. Gu, X. B. Zhao and H. J. Fan, ACS Nano, 2012, 6, 5531 CrossRef CAS PubMed.
- S. Y. Han, D. H. Lee, Y. J. Chang, S. O. Ryu, T. J. Lee and C. H. Chang, J. Electrochem. Soc., 2006, 153, 382 CrossRef.
- T. Fröschl, U. Hörmann, P. Kubiak, G. Kučerová, M. Pfanzelt, C. K. Weiss, R. J. Behm, N. Hüsing, U. Kaiser, K. Landfesterand and M. Wohlfahrt-Mehrens, Chem. Soc. Rev., 2012, 41, 5313 RSC.
- J. Q. Yan, G. J. Wu, N. J. Guan, L. D. Li, Z. X. Li and X. Z. Cao, Phys. Chem. Chem. Phys., 2013, 15, 10978 RSC.
- W. F. Zhang, Y. L. He, M. S. Zhang, Z. Yin and Q. Chen, J. Phys. D: Appl. Phys., 2000, 33, 912 CrossRef CAS.
- N. Mironova-Ulmane, A. Kuzmin, I. Steins, J. Grabis, I. Sildos and M. Pärs, J. Phys.: Conf. Ser., 2007, 93, 012039 CrossRef.
- Q. F. Zhang, E. Uchaker, S. L. Candelariaa and G. Z. Cao, Chem. Soc. Rev., 2013, 42, 3127 RSC.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- J. H. Zhang, J. P. Tu, D. Zhou, H. Tang, L. Li, X. L. Wang and C. D. Gu, J. Mater. Chem. C, 2014, 2, 10409 RSC.
- J. H. Pan, Q. Z. Huang, Z. Y. Koh, D. Neo, X. Z. Wang and Q. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 6292 CAS.
- X. H. Huang, J. P. Tu, X. H. Xia, X. L. Wang, J. Y. Xiang, L. Zhang and Y. Zhou, J. Power Sources, 2009, 188, 588 CrossRef CAS.
- Q. S. Xie, Y. T. Ma, D. Q. Zeng, L. S. Wang, G. H. Yue and D. L. Peng, Sci. Rep., 2015, 5, 8351 CrossRef CAS PubMed.
- H. Long, T. L. Shi, H. Hu, S. L. Jiang, S. Xi and Z. R. Tang, Sci. Rep., 2014, 4, 7413 CrossRef CAS PubMed.
- X. L. Sun, C. L. Yan, Y. Chen, W. P. Si, J. W. Deng, S. Oswald, L. F. Liu and O. G. Schmidt, Adv. Energy Mater., 2014, 4, 1300912 Search PubMed.
- L. P. An, X. P. Gao, G. R. Li, T. Y. Yan, H. Y. Zhu and P. W. Shen, Electrochim. Acta, 2008, 53, 4573 CrossRef CAS.
- W. Wen, J. M. Wu and M. H. Cao, J. Mater. Chem. A, 2013, 1, 3881 CAS.
- U. Boesenberg, M. A. Marcus, A. K. Shukla, T. H. Yi, E. McDermott, P. F. The, M. Srinivasan, A. Moewes and J. Cabana, Sci. Rep., 2014, 4, 7133 CrossRef CAS PubMed.
- L. L. Luo, J. S. Wu, J. M. Xu and V. P. Dravid, ACS Nano, 2014, 8, 11560 CrossRef CAS PubMed.
- J. Liang, H. Hu, H. Park, C. H. Xiao, S. J. Ding, U. Paik and X. W. Lou, Energy Environ. Sci., 2015, 8, 1707 CAS.
- Y. Y. Hu, Z. G. Liu, K. W. Nam, O. J. Borkiewicz, J. Cheng, X. Hua, M. T. Dunstan, X. Q. Yu, K. M. Wiaderek, L. S. Du, K. W. Chapman, P. J. Chupas, X. Q. Yang and C. P. Grey, Nat. Mater., 2013, 12, 1130 CrossRef CAS PubMed.
- F. Lin, D. Nordlund, T. C. Weng, Y. Zhu, C. M. Ban, R. M. Richards and H. L. L. Xin, Nat. Commun., 2014, 5, 3358 Search PubMed.
- M. Khairy and S. A. El-Safty, RSC Adv., 2013, 3, 23801 RSC.
- K. X. Wang, X. H. Li and J. S. Chen, Adv. Mater., 2015, 27, 527 CrossRef CAS PubMed.
- B. Varghese, M. V. Reddy, Z. Yanwu, C. S. Lit, T. C. Hoong, G. V. Subba Rao, B. V. R. Chowdari, T. S. Wee, C. T. Lim and C. H. Sow, Chem. Mater., 2008, 20, 3360 CrossRef CAS.
- J. Y. Liao, D. Higgins, G. Lui, V. Chabot, X. C. Xiao and Z. W. Chen, Nano Lett., 2013, 13, 5467 CrossRef CAS PubMed.
- P. L. Taberna, S. Mitra, P. Poizot, P. Simon and J.-M. Tarascon, Nat. Mater., 2006, 5, 567 CrossRef CAS PubMed.
- H. Han, T. Song, E. K. Lee, A. Devadoss, Y. Jeon, J. Ha, Y. C. Chung, Y. M. Choi, Y. G. Jung and U. Paik, ACS Nano, 2012, 6, 8308 CrossRef CAS PubMed.
- C. Wang, L. X. Wu, H. Wang, W. H. Zuo, Y. Y. Li and J. P. Liu, Adv. Funct. Mater., 2015, 25, 3524 CrossRef CAS.
- L. H. Chu, M. C. Li, X. D. Li, Y. Wang, Z. P. Wan, S. Y. Dou, D. D. Song, Y. F. Li and B. Jiang, RSC Adv., 2015, 5, 4385 RSC.
- M. F. Hassan, M. M. Rahman, Z. P. Guo, Z. X. Chen and H. K. Liu, J. Mater. Chem., 2010, 20, 9707 RSC.
- N. A. Kyeremateng, C. Lebouin, P. Knauth and T. Djenizian, Electrochim. Acta, 2013, 88, 814 CrossRef CAS.
- J. Y. Zhang, J. X. Shen, T. L. Wang, H. Y. Zhang, C. B. Wei, K. C. Zhang and Y. Z. Yue, CrystEngComm, 2015, 17, 1710 RSC.
- M. Osiak, H. Geaney, E. Armstrong and C. O’Dwyer, J. Mater. Chem. A, 2014, 2, 9433 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16894e |
| This journal is © The Royal Society of Chemistry 2015 |