Synthesis and optical properties of composite films from P3HT and sandwich-like Ag–C–Ag nanoparticles
Lingpeng Yanab,
Yamin Haoab,
Xiaoting Fengab,
Yongzhen Yang*ab,
Xuguang Liu*abc,
Yongkang Chenad and
Bingshe Xuab
aKey Laboratory of Interface Science and Engineering in Advanced Materials, Ministry of Education, Taiyuan University of Technology, Taiyuan 030024, China. E-mail: yyztyut@126.com; liuxuguang@tyut.edu.cn
bResearch Center of Advanced Materials Science and Technology, Taiyuan University of Technology, Taiyuan 030024, China
cCollege of Chemistry and Chemical Engineering, Taiyuan University of Technology, Taiyuan 030024, China
dUniversity of Hertfordshire, School of Engineering and Technology, Hatfield, Herts. AL10 9AB, UK
First published on 7th September 2015
Abstract
Sandwich-like Ag–C–Ag nanoparticles (Ag–C–Ag NPs) were synthesized under mild hydrothermal conditions in a one-step method. With this approach, Ag was not only encapsulated in the centre of an individual carbon nanosphere, but was also uniformly dispersed within the carbon matrix up to the sphere's shell. Then, poly(3-hexylthiophene):Ag–C–Ag NPs (P3HT:Ag–C–Ag NPs) composite films were prepared by a spin coating method with a chlorobenzene solution of Ag–C–Ag NPs and P3HT. Both morphology and microstructure of Ag–C–Ag NPs were investigated by field emission scanning electron microscopy and high resolution transmission electron microscopy. The possible formation mechanism was proposed. The results have indicated that the Ag–C–Ag NPs present many functional groups and their energy levels match with those of P3HT. It has been observed that an introduction of Ag–C–Ag NPs to P3HT can induce broad and high-absorbing spectra as well as great photoluminescence quenching of P3HT. It is evident that sandwich-like Ag–C–Ag NPs have a great potential to be a new acceptor material in photovoltaic devices.
1. Introduction
Hybrid nanoparticles or sub-microparticles represent a new type of structure that has attracted considerable attention in recent years. The hybrid structure possesses improved physical and chemical properties over its single-component counterparts1–8 such as remarkably enhanced photoluminescence efficiency and prominent catalytic properties in specific reactions, which may widen its potential application to electronics, magnetics, optics, and catalysis.Metal materials have also been reported to increase light absorption in active layers of solar cells (SCs) through their plasma effects.9–11 At the nano-scale, metallic nanoparticles can be penetrated by electromagnetic waves, which induce a separation of charges, and consequently a coherent oscillation (surface plasmon). Surface plasmons are essentially light waves that are trapped on the surface because of their interaction with the free electrons of the metal.12 The primary consequences of excitation due to localized surface plasmon resonances are selective photo absorption, scattering, and local electromagnetic field enhancements.13 Silver (Ag) exhibits the highest electrical and thermal conductivities among all types of metal and has the good property of oxidation resistance. Its optical trapping potential is also most effective owing to its high scattering efficiency in the visible range.14 However, silver nanoparticles are prone to coalesce as a result of van der Waals forces and high surface energies unless they are protected.2 Carbon nanospheres have been proposed to provide that role in protection.15 As a part of the carbon family, carbon nanospheres, which have stable chemical and thermal properties, as well as low preparation costs, are promising candidates to be used as metal supports.
Sun and Li16 prepared Ag/C core/shell spheres using water as an environmentally benign solvent. Carbon nanospheres loaded with silver nanoparticles were also obtained by two-step methods17,18. Though Wang et al.17 investigated a new synthesis route for Ag/C core/shell nanospheres with many small Ag particles on the surface of the shell structure, the synthesis steps were complex and not so eco-friendly.
In this study, sandwich-like Ag–C–Ag nanoparticles (Ag–C–Ag NPs), with Ag encapsulated in the middle of a carbon nanosphere distributed in the carbon shell and loaded on the surface of the carbon sphere, were synthesized by a one-step method under typical hydrothermal conditions. Their application as an acceptor for polymer solar cells with P3HT as the donor becomes attractive in this study. This composite structure is expected to yield an enhanced and broader absorption range of the solar spectrum that could induce a remarkable increase in charge generation. Ultraviolet-visible (UV-Vis) and photoluminescence (PL) spectroscopies were used to confirm the optical behaviour of the obtained products.
2. Experimental
2.1 Preparation of sandwich-like Ag–C–Ag NPs
In a typical preparation, 30 ml of 0.4 M aqueous glucose solution was added to a 50 ml Teflon liner, then 2 ml of 0.03 M AgNO3 aqueous solution was added dropwise with vigorous stirring. After subjecting to ultrasonication for 20 minutes, the mixture was placed in an autoclave. The autoclave was heated to a certain temperature (180, 190 and 200 °C), kept at this selected temperature for 4 hours, and then cooled to room temperature. The resulting salmon colored precipitate was collected through centrifugation and washed several times with distilled water and absolute alcohol. The product was redispersed in absolute ethanol and subsequently dried at 50 °C for 24 hours in an oven.2.2 Fabrication of P3HT:Ag–C–Ag NPs composite films
Regioregular P3HT was provided commercially by Luoyang Microlight Material Technology Co., Ltd and used without further purification. 10 mg of P3HT was diluted in 1 ml of chlorobenzene; then, 0, 2.5, 5 and 10 mg of Ag–C–Ag NPs were added in the abovementioned solutions. P3HT and Ag–C–Ag NPs in the mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 4
0, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed in the solution state. Subsequently, the mixtures were magnetically stirred for 12 hours.
1 were mixed in the solution state. Subsequently, the mixtures were magnetically stirred for 12 hours.
Glass substrates were pre-cleaned repeatedly in an ultrasonic bath and sequentially cleaned by detergent, deionized water, ethanol and acetone. P3HT:Ag–C–Ag NPs solution was spin-coated onto a cleaned glass substrate at a spin rate of 1000 rpm for 1 minute and dried at room temperature.
2.3 Characterization of materials
The morphologies of the samples were characterized by a JSM-6700F field emission scanning electron microscope (FESEM) at 10 kV at an acceleration voltage range of 0.5–30 kV and a beam intensity range of 10−13–2 × 10−9 A. The samples were coated with gold (Au) in an evaporator to avoid charging during electron irradiation. The structures of the products were observed with a JEM-2010 high resolution transmission electron microscope (HRTEM), a Tensor 27 Fourier transformation infrared spectrometer (FTIR) and a D8 Advance X-ray diffractometer (XRD). The thermal stability of the samples was characterized by thermogravimetry (Netzsch TG 209F3) operating in air atmosphere from 100 °C to 900 °C at a heating rate of 10 °C min−1. To obtain the electronic structure of the Ag–C–Ag NPs, a cyclic voltammetry (CV) analysis was conducted with a CS350 electrochemical workstation using a Pt disk, Pt wire and calomel electrode as the working electrode, counter electrode and reference electrode, respectively, and using tetrabutylammonium perchlorate (TBAP, 0.1 M) dimethyl formamide as the supporting electrolyte. U-3900 ultraviolet-visible spectrophotometer (UV-Vis) and Fluoromax-4 photoluminescence spectrometer (PL) were employed to characterize the optical properties of the composites.3. Results and discussion
3.1 Characterization of sandwich-like Ag–C–Ag NPs
Ag–C–Ag NPs were prepared by the hydrothermal method at different temperatures, as shown in Fig. 1. It can be seen from the TEM images of the samples that the as-synthesized products have different morphologies and structures. The carbon nanospheres were severely agglomerated and contained barely any Ag nanoparticles, as shown in Fig. 1(a). This suggests that Ag+ did not participate in a chemical reaction at the low temperature of 180 °C. As shown in Fig. 1(b), when the temperature was 190 °C, typical sandwich-like Ag–C–Ag NPs were formed and the Ag nanoparticles were encapsulated in the middle of the carbon spheres, distributed in the carbon shell and loaded on the surfaces of the carbon spheres. This illustrates that Ag+ was gradually reduced into Ag nanoparticles at the temperature of 190 °C, and the Ag nanoparticles were also spread within the carbon nanospheres in addition to Ag nanoparticles being encapsulated in the carbon spheres. However, Ag+ was reduced rapidly at the temperature of 200 °C, and Ag nanoparticles were only encapsulated in the carbon spheres, as shown in Fig. 1(c). In general, the optimum temperature for obtaining Ag–C–Ag NPs is 190 °C.Fig. 2(a) presents a typical FESEM image of Ag–C–Ag NPs, which exhibit regular spherical shapes with diameters in the range from 300 to 520 nm. Energy dispersive spectroscopy (EDS) indicates that the sandwich-like structure consists of C, O and Ag, as shown in the top right corner of Fig. 2(a). To further investigate the detailed structure of the products, TEM analyses were conducted. First, it can be distinctly seen from Fig. 2(b) that Ag particles (arrow A) are encapsulated in the carbon shell, and the diameter of the core and the thickness of the shell are about 80 and 200 nm, respectively. Second, Fig. 2(b) also shows that the Ag nanoparticles (about 20 nm in size) that are dispersed in the carbonaceous matrix can be divided into two parts with different distributions. The first part is on the surface of the carbon shell with the crystal spacing of 0.23 nm that corresponds to Ag (111), as marked by the square in Fig. 2(b) and shown in an inset at the bottom left corner of Fig. 2(c). As shown in an inset at the top left corner of Fig. 2(c), the electron diffraction pattern indicates the single-crystalline nature of the Ag nanoparticles. It can also be observed that a thin layer of carbon (less than 2 nm) surrounds the Ag nanocrystals. The second part is dispersed inside the carbon matrix (arrow B in Fig. 2(b)). Third, a small amount of hollow spheres without an Ag nanoparticle core or with a split Ag nanoparticle core was found, as marked with the circles in Fig. 2(b) and (d). More importantly, compared with the carbon spheres with a full Ag nanoparticle core, these hollow spheres without an Ag nanoparticle core or with a split Ag nanoparticle core have more Ag nanoparticles dispersed in the carbon matrix.
Fig. 3 presents the XRD profiles of Ag–C–Ag NPs. It can be seen that there is a broad peak in the range of 15–30° that is attributed to the (002) reflection of a carbon layer in Ag–C–Ag NPs, indicating the existence of amorphous carbon. It is found that strong diffraction peaks assigned to Ag nanocrystals at 8.15°, 44.30°, 64.45° and 77.30° are associated with the (111), (200) (220) and (311) reflections of Ag nanocrystals, respectively, illustrating that the Ag nanocrystals are stacked in commonly known face-centred cubic structure. Otherwise, the strongest diffraction peak of Ag nanocrystals is the (111) reflection, indicating that the preferable growth face of Ag nanocrystals is in (111) plane.
To determine the ratio of Ag nanocrystals in Ag–C–Ag NPs and the thermal stability of Ag–C–Ag NPs, a TG test in air was carried out. As can be seen in Fig. 4, Ag–C–Ag NPs exhibit only 5.50% of weight loss when the temperature is increased up to 275 °C. When the temperature exceeded 285 °C, the Ag–C–Ag NPs began to lose weight rapidly and reached a steady state above 380 °C. During the period of fast weight loss, carbon plies were oxidized to carbon dioxide, and eventually the oxidization and deoxidization of Ag nanocrystals was in equilibrium. The final product was Ag nanocrystals because Ag2O is unstable at high temperatures. Thus, the ratio of Ag nanocrystals in Ag–C–Ag NPs is about 5.30%.
FTIR measurements were carried out to investigate the functional groups of products after the hydrothermal treatment. As shown in Fig. 5, the bands at 1701 and 1625 cm−1 were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations, respectively, indicating the aromatization of glucose during the hydrothermal treatment. It can be seen that the as-synthesized Ag–C–Ag NPs exhibited a strong band of –OH (O–H stretching vibrations) at 3429 cm−1, along with the weak peaks of C–OH at 1385 and 1302 cm−1, which means that the as-synthesized Ag–C–Ag NPs are rich in hydroxyl groups. The presence of a functional group makes Ag–C–Ag NPs disperse better in aqueous medium and common organic solvents, which can greatly widen the range of their applications.
C vibrations, respectively, indicating the aromatization of glucose during the hydrothermal treatment. It can be seen that the as-synthesized Ag–C–Ag NPs exhibited a strong band of –OH (O–H stretching vibrations) at 3429 cm−1, along with the weak peaks of C–OH at 1385 and 1302 cm−1, which means that the as-synthesized Ag–C–Ag NPs are rich in hydroxyl groups. The presence of a functional group makes Ag–C–Ag NPs disperse better in aqueous medium and common organic solvents, which can greatly widen the range of their applications.
3.2 Formation mechanism of sandwich-like Ag–C–Ag NPs
A possible formation mechanism is schematically illustrated in Fig. 6. There are four stages. Initially, Ag+ did not react with glucose under the ambient conditions. However, as the solution was sealed in autoclaves and heated to 190–200 °C, Ag+ was reduced by glucose under hydrothermal conditions. At the beginning stage, as shown in Fig. 6(a), most of the silver ions in the solution was deoxidized by glucose and Ag nanoparticles were nucleated.7,16 In the following stage, as shown in Fig. 6(b), carbon shells were formed by the in situ deposition of carbonaceous products around the surface of an Ag core with Ag nanoparticles, catalyzing the carbonization of glucose.7,19 In the third stage, as shown in Fig. 6(c), as the reaction proceeded, unreacted Ag+ in the reaction system was gradually reduced into Ag nanoparticles. The Ag nanoparticles were evenly dispersed in the solution system with reactive surfaces, which catalyzed the following carbonization of glucose and led to gradual embedding in the carbon matrix of the Ag nanoparticles along with the carbonization of glucose, as indicated by the TEM images of Ag–C–Ag NPs marked with arrow B in Fig. 2(b). In the final stage, as shown in Fig. 6(d), Ag nanoparticles loaded on the surface of a carbon shell (as marked with square in Fig. 2(b)) were obtained by the subsequent reduction of redundant Ag+.Based upon an analysis of the Zeta potential on the products, the surfaces of the Ag–C–Ag NPs have been negatively charged in water (pH = 7, ξ = −25 eV). This negative charge originated from the ionization of –COOH and –OH groups on the surface of Ag–C–Ag NPs.20 A small amount of hollow or split nanoparticles, as marked by the circles in Fig. 2(b) and (d), may be induced by the electrostatic suction between a Ag core with positive electricity and a carbon matrix.19 Under the action of electrostatic attraction, Ag nanoparticles were diffused from the core into the carbonaceous matrix, resulting in the splitting of the Ag core and the presence of trace amounts of hollow or split Ag nanoparticles, as shown in Fig. 6(e) and (f). Thus, it can be reasonably suggested that some of the Ag nanoparticles dispersed in the carbon matrix and on the surface of carbon shell originated from the outward diffusion of Ag nanoparticles through electrostatic forces. This is also verified by the fact that carbon spheres with a hollow or split Ag nanoparticle core have more Ag nanoparticles dispersed in the carbon matrix than the carbon spheres with full Ag cores, as shown in the circles of Fig. 2(b) and (d). Therefore, it is suggested that the formation of Ag nanoparticles dispersed in the carbon matrix and on the surface of the carbon shell may result from two reasons: a reduction of Ag+ and outward diffusion of the Ag nanoparticles under electrostatic forces.
3.3 Feasibility analysis of a potential application
To explore a potential application of the Ag–C–Ag NPs and Ag nanoparticles encapsulated in carbon spheres (Ag@C NPs) in photovoltaic cells, a CV test of Ag–C–Ag NPs and Ag@C NPs was carried out for determination of their band gap energies. As shown in Fig. 7, both HOMO and LUMO energy levels were estimated from their onset oxidation potential and reduction potential according to eqn (1) and (2) (ref. 21) as follows:| HOMO = −(Eox0 + 4.74) (eV), | (1) |
| LUMO = −(Ered0 + 4.74) (eV), | (2) |
It can be seen in Fig. 7 that Eox0 and Ered0 of Ag@C NPs are 0.95 V and −0.06 V, respectively. Therefore, the calculated HOMO and LUMO energy levels are −5.69 and −4.68 eV, respectively. Eg, the difference between HOMO and LUMO energy levels, is calculated to be 1.01 eV. Similarly, the HOMO level, LUMO level and Eg of Ag–C–Ag NPs are calculated to be −5.55, −4.40 and 1.15 eV, respectively. Free holes and electrons cannot be created directly in composite films when light is absorbed because excitons have a large binding energy of 0.3 eV;22 thus, the energy gaps of electron donors and electron acceptors ΔE1 and ΔE3 should be greater than 0.3 eV, where ΔE1 = DLUMO − ALUMO and ΔE3 = DHOMO − AHOMO; D refers to electron donors and A refers to electron acceptors. It has been reported that the HOMO and LUMO of P3HT are −5.0 and −3.0 eV,19 respectively. As a result, ΔE1 and ΔE3 of the active layer based on electron-donating P3HT and electron-accepting Ag@C NPs are 0.69 and 1.68 eV, respectively, and those of the active layer based on electron-donating P3HT and electron-accepting Ag–C–Ag NPs are 0.55 and 1.40 eV, respectively. Both are greater than the exciton binding energy. Therefore, it is suggested that Ag@C NPs and Ag–C–Ag NPs could be suitable to be used as the acceptor materials of polymer solar cells. For donor–acceptor bulk heterojunction solar cells, the Voc value is mainly determined by the difference between the acceptor's LUMO level and the donor's HOMO level,23 which can be expressed as follows:
| Voc = e−1 × (|HOMOdonor| − |LUMOacceptor| − 0.3 eV) | (3) |
In the active layers, the HOMO level for P3HT is −5.0 eV, the LUMO levels of Ag@C NPs and Ag–C–Ag NPs are −4.68 and −4.40 eV, respectively. Thus, the Voc values of P3HT:Ag@C and P3HT:Ag–C–Ag NPs are determined to be 0.32 and 0.60 V, respectively, according to eqn (3). It can be suggested from the Voc values of P3HT:Ag@C and P3HT:Ag–C–Ag NPs that Ag–C–Ag NPs are more suitable as active materials for photovoltaic cells.
3.4 Optical properties of P3HT:Ag–C–Ag NPs composite films
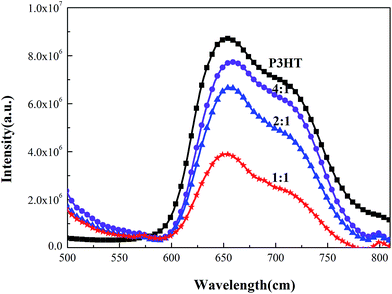
To further investigate a possible application of Ag–C–Ag NPs in an active layer, the P3HT:Ag–C–Ag NPs solutions were spin-coated on quartz and characterized by UV-Vis and PL. The PL spectra of pure P3HT, Ag–C–Ag NPs and composite films with different mass ratios were studied, with emphasis on quenching phenomena to compare their efficiencies for a photo-induced charge transfer. The PL spectra were obtained at an excitation wavelength of 420 nm in the range from 500 to 820 nm. It is evident from Fig. 8 that when Ag–C–Ag NPs were introduced into the P3HT, the PL intensity of the composite films was significantly quenched. This quenching suggests a photo-induced charge transfer in the composite films.24 This implies that electrons and holes are separated effectively and transferred in their interpenetrating phase. The quenching phenomena are more obvious when the mixing ratios are 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Fig. 8 also shows a few minor peaks that indicate that some of the excited electrons of P3HT were radiatively decayed without a completion of a charge transfer to the acceptor.25 The outcome from the PL quenching test suggests that the Ag–C–Ag NPs may accept electrons efficiently from the P3HT donor polymer.
1. Fig. 8 also shows a few minor peaks that indicate that some of the excited electrons of P3HT were radiatively decayed without a completion of a charge transfer to the acceptor.25 The outcome from the PL quenching test suggests that the Ag–C–Ag NPs may accept electrons efficiently from the P3HT donor polymer.
Fig. 9 shows the morphology of a typical P3HT:Ag–C–Ag NPs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; w/w) composite film. As can be seen from the TEM image of the composite film, the sandwich-like Ag–C–Ag NPs can also been observed clearly, which is consistent with the TEM image of Ag–C–Ag NPs, and they have a good dispersion in P3HT, attributed to the good solubility of Ag–C–Ag NPs in chlorobenzene. This good dispersion contributes to the formation of an interpenetrating network structure in the composite film, which is conducive to the efficient transfer of photo-induced charge in the composite film. This result is in good agreement with the PL characterization results shown in Fig. 8.
1; w/w) composite film. As can be seen from the TEM image of the composite film, the sandwich-like Ag–C–Ag NPs can also been observed clearly, which is consistent with the TEM image of Ag–C–Ag NPs, and they have a good dispersion in P3HT, attributed to the good solubility of Ag–C–Ag NPs in chlorobenzene. This good dispersion contributes to the formation of an interpenetrating network structure in the composite film, which is conducive to the efficient transfer of photo-induced charge in the composite film. This result is in good agreement with the PL characterization results shown in Fig. 8.
To investigate the optical properties of P3HT:Ag–C–Ag NPs composite films, the UV-Vis absorption spectra of P3HT:Ag–C–Ag NPs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; w/w) were obtained. Besides, Ag–C–Ag NPs prepared at 180 °C (Ag–C–Ag NPs-180) were used for a comparison, which contain barely any Ag nanoparticles. Fig. 10 shows the UV-Vis absorption spectra of P3HT, P3HT:Ag–C–Ag NPs (2
1; w/w) were obtained. Besides, Ag–C–Ag NPs prepared at 180 °C (Ag–C–Ag NPs-180) were used for a comparison, which contain barely any Ag nanoparticles. Fig. 10 shows the UV-Vis absorption spectra of P3HT, P3HT:Ag–C–Ag NPs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; w/w), P3HT:Ag–C–Ag NPs-180 (2
1; w/w), P3HT:Ag–C–Ag NPs-180 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; w/w) and the difference spectra between the composite films and P3HT in the solid state. In the spectrum of pure P3HT, the highest energy peak exists at 526 nm. Two shoulders can also be found at 553 and 599 nm, which represent the vibrational excitations of crystalline P3HT. After pure P3HT is blended with the P3HT:Ag–C–Ag NPs-180, the absorbance of the composite film in the region of 400–700 nm is weakly increased, because the Ag–C–Ag NPs-180 exhibits a weak absorption. However, after the introduction of Ag–C–Ag NPs, the absorption of P3HT:Ag–C–Ag NPs composite films show an obvious enhancement in the range of 300–650 nm, which is likely attributed to the plasma resonances of Ag–C–Ag NPs. In addition, by comparing the difference spectra of P3HT with P3HT:Ag–C–Ag NPs and P3HT:Ag–C–Ag NPs-180, the stronger absorption of P3HT:Ag–C–Ag NPs can also be revealed.
1; w/w) and the difference spectra between the composite films and P3HT in the solid state. In the spectrum of pure P3HT, the highest energy peak exists at 526 nm. Two shoulders can also be found at 553 and 599 nm, which represent the vibrational excitations of crystalline P3HT. After pure P3HT is blended with the P3HT:Ag–C–Ag NPs-180, the absorbance of the composite film in the region of 400–700 nm is weakly increased, because the Ag–C–Ag NPs-180 exhibits a weak absorption. However, after the introduction of Ag–C–Ag NPs, the absorption of P3HT:Ag–C–Ag NPs composite films show an obvious enhancement in the range of 300–650 nm, which is likely attributed to the plasma resonances of Ag–C–Ag NPs. In addition, by comparing the difference spectra of P3HT with P3HT:Ag–C–Ag NPs and P3HT:Ag–C–Ag NPs-180, the stronger absorption of P3HT:Ag–C–Ag NPs can also be revealed.
 | ||
Fig. 10 The UV-Vis spectra of P3HT, P3HT:Ag–C–Ag NPs (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w:w) and P3HT:Ag–C–Ag NPs-180 composite film (2 1, w:w) and P3HT:Ag–C–Ag NPs-180 composite film (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w:w). 1, w:w). | ||
To study the plasmon resonance of Ag–C–Ag NPs, the UV-Vis absorption spectra of Ag–C–Ag NPs (5 mg mL−1, 1000 rpm) and Ag–C–Ag NPs-180 (5 mg mL−1, 1000 rpm) in solid state were characterized, as shown in Fig. 11. In comparison with Ag–C–Ag NPs-180, the Ag–C–Ag NPs show much more intensive light absorption bands in the range of 300–650 nm, which is consistent with the difference spectra of P3HT:Ag–C–Ag NPs and P3HT, suggesting the existence of plasmons in the P3HT:Ag–C–Ag NPs film. Silver nanoparticles can intensely absorb visible light because of the surface plasmon resonance effect;26 thus, the Ag–C–Ag NPs show more intensive light absorption than Ag–C–Ag NPs-180. The spectral enhancement is likely due to two major factors. The first could be a local enhancement of the electromagnetic field on an account of localized surface plasmon resonances.27,28 The other could be plasmon resonance scattering, which becomes dominant when a larger Ag core is used. It appears that the scattering lengthens the optical path several times in the active layer, thereby enhancing the degree of light absorption.29
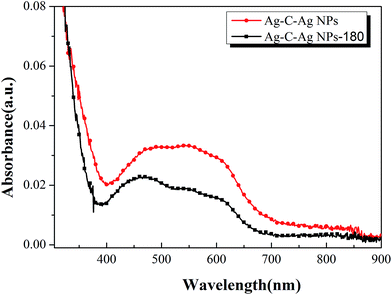 | ||
| Fig. 11 The UV-Vis spectra of Ag–C–Ag NPs (5 mg mL−1, 1000 rpm) and Ag–C–Ag NPs-180 (5 mg mL−1, 1000 rpm). | ||
4. Conclusions
Synthesis and optical properties of composite films from P3HT and sandwich-like Ag–C–Ag nanoparticles have been investigated. Based on the abovementioned results and discussion, the following conclusions can be drawn:(i) Sandwich-like Ag–C–Ag NPs were hydrothermally synthesized by a one-step method and the possible formation mechanism of Ag–C–Ag NPs was proposed. With this method, Ag was not only encapsulated in the centre of a carbon nanosphere, but was also uniformly dispersed within the carbon matrix and on the carbon nanosphere's shell.
(ii) The ratio of Ag nanocrystals in Ag–C–Ag NPs is about 5.30% and the Ag–C–Ag NPs present many functional groups. These groups facilitate further functionalization for various applications.
(iii) With a matched energy level, the Ag–C–Ag NPs and P3HT composite films possess apparent quenching phenomena, indicating an efficiently photo-induced charge transfer in the composite films, and enhanced absorption in the optical range, which is due to the plasmon resonances of Ag nanoparticles in Ag–C–Ag NPs. Therefore, sandwich-like Ag–C–Ag NPs have a great potential to be a new and highly efficient acceptor material for application in polymer solar cells.
Conflict of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences for high resolution transmission electron microscopy test. The authors acknowledge financial support from National Natural Science Foundation of China (21176169), Shanxi Provincial Key Innovative Research Team in Science and Technology (2012041011), International Science & Technology Cooperation Program of China (2012DFR50460), Research Project Supported by Shanxi Scholarship Council of China (2012-038), Post-graduate Innovation Program of Shanxi Province (20143010), Post-graduate Innovation Foundation of Taiyuan University of Technology (S2014103) and Open Foundation project of Key Laboratory of Interface Science and Engineering in Advanced Materials (Taiyuan University of Technology) (KLISEAM201502).Notes and references
- J. Cheng, Y. Wang, C. Teng, Y. Shang, L. Ren and B. Jiang, Chem. Eng. J., 2014, 242, 285–293 CrossRef CAS PubMed.
- J. Lee, K. Han and D. J. Jang, Appl. Catal., A, 2014, 469, 380–386 CrossRef CAS PubMed.
- V. K. Gupta, M. L. Yola, T. Eren, F. Kartal, M. O. Çağlayan and N. Atar, J. Mol. Liq., 2014, 190, 133–138 CrossRef CAS PubMed.
- M. K. Joshi, H. R. Pant, H. J. Kim, J. H. Kim and C. S. Kim, Colloids Surf., A, 2014, 446, 102–108 CrossRef CAS PubMed.
- S. Yang, C. Nie, H. Liu and H. Liu, Mater. Lett., 2013, 100, 296–298 CrossRef CAS PubMed.
- S. Mao, W. Li, Y. Long, Y. Tu and A. Deng, Anal. Chim. Acta, 2012, 738, 35–40 CrossRef CAS PubMed.
- S. Y. Wu, Y. S. Ding, X. M. Zhang, H. O. Tang, L. Chen and B. X. Li, J. Solid State Chem., 2008, 181, 2171–2177 CrossRef CAS PubMed.
- Y. D. Han, J. W. Lee, D. H. Park, S. H. Yang, B. K. Kim, J. Kim and J. Joo, Synth. Met., 2011, 161, 2103–2106 CrossRef CAS PubMed.
- L. Chen, S. Zhang, L. Chang, L. Zeng, X. Yu, J. Zhao, S. Zhao and C. Xu, Electrochim. Acta, 2013, 93, 293–300 CrossRef CAS PubMed.
- V. E. Ferry, J. N. Munday and H. A. Atwater, Adv. Mater., 2010, 22, 4794–4808 CrossRef CAS PubMed.
- H. A. Atwater and A. Polman, Nat. Mater., 2010, 9, 205–213 CrossRef CAS PubMed.
- W. L. Barnes, A. Dereux and T. W. Ebbesen, Nature, 2003, 424, 824–830 CrossRef CAS PubMed.
- T. R. Jensen, M. D. Malinsky, C. L. Haynes and R. P. Van Duyne, J. Phys. Chem. B, 2000, 104, 10549–10556 CrossRef CAS.
- X. Li, W. C. H. Choy, H. Lu, W. E. Sha and A. H. P. Ho, Adv. Funct. Mater., 2013, 23, 2728–2735 CrossRef CAS PubMed.
- J. Cui, C. Hu, Y. Yang, Y. Wu, L. Yang, Y. Wang, Y. Liu and Z. Jiang, J. Mater. Chem., 2012, 22, 8121–8126 RSC.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- H. Wang, Q. Jin, L. Yang and Y. Liu, J. Nanopart. Res., 2013, 15, 1–9 Search PubMed.
- Y. Zhao, Z. Q. Wang, X. Zhao, W. Li and S. X. Liu, Appl. Surf. Sci., 2013, 266, 67–72 CrossRef CAS PubMed.
- C. Li, Y. Zhu, X. Zhang, X. Yang and C. Li, RSC Adv., 2012, 2, 1765–1768 RSC.
- B. Zhang, C. Y. Liu and Y. Liu, Eur. J. Inorg. Chem., 2010, 2010, 4411–4414 CrossRef PubMed.
- Y. Li, L. Yan, Y. Yang, X. Liu and B. Xu, J. Mater. Res., 2014, 29, 492–500 CrossRef CAS.
- J. Subbiah, C. M. Amb, I. Irfan, Y. Gao, J. R. Reynolds and F. So, ACS Appl. Mater. Interfaces, 2012, 4, 866–870 CAS.
- Y. Li, Y. Hu, Y. Zhao, G. Shi, L. Deng, Y. Hou and L. Qu, Adv. Mater., 2011, 23, 776–780 CrossRef CAS PubMed.
- S. M. Abdullah, Z. Ahmad, F. Aziz and K. Sulaiman, Org. Electron., 2012, 13, 2532–2537 CrossRef CAS PubMed.
- H. U. Kim, D. Mi, J. H. Kim, J. B. Park, S. C. Yoon, U. C. Yoon and D. H. Hwang, Sol. Energy Mater. Sol. Cells, 2012, 105, 6–14 CrossRef CAS PubMed.
- S. Sun, W. Wang, L. Zhang, M. Shang and L. Wang, Catal. Commun., 2009, 11, 290–293 CrossRef CAS PubMed.
- I. Ding, J. Zhu, W. Cai, S. J. Moon, N. Cai, P. Wang, S. M. Zakeeruddin, M. Grätzel, M. L. Brongersma and Y. Cui, Adv. Energy Mater., 2011, 1, 52–57 CrossRef CAS PubMed.
- S. Pillai and M. Green, Sol. Energy Mater. Sol. Cells, 2010, 94, 1481–1486 CrossRef CAS PubMed.
- J. L. Wu, F. C. Chen, Y. S. Hsiao, F. C. Chien, P. Chen, C. H. Kuo, M. H. Huang and C. S. Hsu, ACS Nano, 2011, 5, 959–967 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |