From fish scales to highly porous N-doped carbon: a low cost material solution for CO2 capture
Bicheng Huanga,
Hongyuan Shaoa,
Naiqiang Liua,
Zhichuan J. Xu*b and
Yaqin Huang*a
aBeijing Laboratory of Biomedical Materials, Beijing University of Chemical Technology, Beijing 10029, China. E-mail: huangyq@mail.buct.edu.cn; Tel: +86-13911551112
bSchool of Materials Science and Engineering, Nanyang Technological University, 50 Nanyang Avenue, Singapore 639798, Singapore. E-mail: xuzc@ntu.edu.sg; Tel: +65-65923170
First published on 30th September 2015
Abstract
This article reports a strategy to use fish scales as raw materials for synthesizing CO2 capture materials. The synthesis employs thermal and chemical treatment to convert fish scales into N-rich porous carbons. The proteins in the fish scales are the major source of carbon and nitrogen. By varying the reaction conditions, the porosity and N content can be controlled in the produced porous carbons. It was found that the porosity first increases and then decreases with an increase in thermal treatment temperature; the N content decreases with an increase in the temperature. The capture capacity of the as-synthesized carbon (NFPC-750) for CO2 can be up to 171 mg g−1 at 25 °C, 1 bar. This high capacity is attributable to its porous structure with a high specific surface area (up to 3206 m2 g−1) and large pore volume (micropore volume up to 0.76 cm3 g−1 and total pore volume up to 2.29 cm3 g−1). More attractively, quaternary nitrogen is effectively preserved (2.90% N), which should be another contributor to enhance the CO2 capture capacity through the chemical adsorption between nitrogen groups and CO2. In addition, the sorbent preliminarily exhibits high cycle stability with retention of 91.8% of its initial CO2 capacity after 10 cycles. This highly porous N-doped porous carbon obtained from fish scales is thus considered a promising material for CO2 capture.
1. Introduction
Carbon dioxide (CO2) is one of the major greenhouse gases. Its amount in the atmosphere has been increasing in recent decades and is believed to be the major factor resulting in global warming. To reduce the amount of CO2, efforts have been made to reduce emission at sources such as power plants as well as develop new techniques to capture and then reuse this small molecule. In recent years, various solid sorbents have been developed; popular materials include zeolite, hydrotalcite-like compounds, and carbon-based sorbents such as carbon nanotubes and porous carbons.1–9 The CO2 capture capacities of these sorbents mainly originate from their high specific surface areas. To further increase the capture capacity, basic sites are introduced into the sorbents to enhance the affinity of CO2 for the surface.10,11Nitrogen-containing groups have been reported to act as basic sites in porous carbons for CO2 adsorption;12–17 therefore, there is a research interest based on developing approaches to introduce N into porous carbons. The earlier reported methods included grafting some N-rich molecules onto the surface of carbons.13,14 In later efforts, some researchers selected carbon precursors with an abundance of nitrogen-containing compounds to facilitate the synthesis of efficient sorbents.2,18–20
In this study, we report for the first time that fish scales can be an excellent raw material for synthesizing highly porous N-doped carbon for CO2 adsorption, with the following advantages. First, protein is the main component of fish scales, containing both carbon and nitrogen. Second, hydroxyapatite, which exists in fish scales on the nanoscale, could be used as a natural template for porous carbon synthesis. Third, fish scales are an inexpensive by product of the food processing industry.21 The results show that the as-synthesized carbon has a very high specific surface area of 3206 m2 g−1 and large pore volume (total pore volume up to 2.29 cm3 g−1). More importantly, this carbon exhibits strong capture capacity for CO2, up to 171 mg g−1 at 25 °C, 1 bar, and is a promising adsorbent for CO2 capture.
2. Experimental method
2.1 Materials preparation
Tilapia fish scales were obtained from Dali, Yunnan province. Clean and dry fish scales were precarbonized at 330 °C for 3 h in a N2 atmosphere. The precarbonized fish scales were then blended with KOH (as an activating agent) in a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and ground to powder. The powder was heated in a N2 atmosphere at different temperatures (in the range of 600–900 °C) for 1 h. For comparison, some precarbonized fish scale powder was directly heated at the same temperatures without KOH. All the activated and non activated products were first washed with 1 M HCl and then washed and filtered with hot deionized water until the electrical conductivity of the filtered liquid was equal to that of pure water. The products were then dried at 100 °C for 24 h to obtain N-doped fish-scale-based porous carbons. In this study, the carbon materials with and without KOH activation are denoted as NFPC-T and NFPC-T-NA, respectively, where “T” represents the carbonization temperature of the material and “NA” implies no activation.
1 and ground to powder. The powder was heated in a N2 atmosphere at different temperatures (in the range of 600–900 °C) for 1 h. For comparison, some precarbonized fish scale powder was directly heated at the same temperatures without KOH. All the activated and non activated products were first washed with 1 M HCl and then washed and filtered with hot deionized water until the electrical conductivity of the filtered liquid was equal to that of pure water. The products were then dried at 100 °C for 24 h to obtain N-doped fish-scale-based porous carbons. In this study, the carbon materials with and without KOH activation are denoted as NFPC-T and NFPC-T-NA, respectively, where “T” represents the carbonization temperature of the material and “NA” implies no activation.
2.2 Materials characteristics
The porous texture of the samples was examined using N2/77 K adsorption/desorption isotherms (Quantachrome NOVA 1200). The surface areas were calculated using the Brunauer–Emmett–Teller (BET) method based on N2 adsorption on the sample. The total pore volumes were estimated to be the volumes of liquid nitrogen adsorbed at P/P0 = 0.99. The micropore volumes were determined by the t-plot method. The pore size distribution plots were extracted from the adsorption branch of the isotherm based on density functional theory. Scanning electron microscopy (SEM, HITACHI S-4700) was used to observe the microstructure of the samples. X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific USA ESCALAB-250) was carried out to determine the N contents of the samples. Fourier transform infrared spectroscopy (FTIR, Thermo Fisher Scientific USA, Nicolet iS5) was used to demonstrate the functional groups of the different samples.2.3 Carbon dioxide adsorption
A CO2 adsorption test was carried out under pure CO2 at 1 bar using a thermogravimetric analyzer (TGA, Hengjiu China). In the experiment, samples in the range of 3–5 mg were loaded into the TGA. Prior to the measurement, the samples were all degassed under N2 at 200 °C for 1 h to remove the impurities. After cooling the sample to the analysis temperature (25 °C or 50 °C), the test began and CO2 was instilled for 1 h to fill the TGA with CO2. After the adsorption test, materials were desorbed under N2 at 200 °C for 1 h. Desorbed materials were used in the cycle test of the CO2 adsorption. The cycle test was performed under the same conditions as the adsorption/desorption test. Adsorption capacity was calculated from the result of the TGA by converting the gross increasing mass into per gram of increasing mass. Selectivity and isosteric heat of adsorption were measured using N2 and CO2 adsorption isotherms at 0 °C and 25 °C (Quantachrome Autosorb-1).3. Results and discussion
3.1 Composition of the synthesized carbons
The precarbonized material (heated to 330 °C) has high N content up to 13.02% and could be a potential raw material for N-rich carbons. The nitrogen contents of the as-synthesized materials are listed in Table 1. The nitrogen content of the samples decreases as the temperature increases, irrespective of whether the sample was activated or not. This is probably because nitrogen is less stable than carbon at high temperatures. In addition, each NFPC-T-NA sample shows a higher N content than the NFPC-T sample treated at the same temperature, which indicates that KOH activation accelerates the N removal. This is consistent with the literature and such activation has been ascribed to the oxidization effect of KOH on N groups.19| Synthesis temperature (°C) | N content (wt%) | |
|---|---|---|
| Activated samples | Not activated samples | |
| a The N content of the precarbonized tilapia fish scales (heated to 330 °C) is 13.02 wt%. | ||
| 600 | 7.49 | 10.80 |
| 750 | 2.90 | 9.23 |
| 900 | 1.50 | 3.79 |
The N 1s core-level spectra of the samples are shown in Fig. 1(a) and (b). In these two figures, the three peaks at 398.7 eV, 400 eV and 400.7 eV correspond to pyridinic nitrogen, pyrolic nitrogen/pyridonic nitrogen, and quaternary nitrogen, respectively.22 It should be noted that pyrolic N and pyridonic N cannot be distinguished by XPS.19 Owing to the oxidization effect of KOH, the 400 eV peak corresponds to pyridonic N, as shown in Fig. 1(a). The pyrolic N/pyridonic N peak shows the lowest stability among the three N species under pyrolytic conditions (Fig. 1(b)), which suggests that the 400 eV peak is more likely due to pyridonic N. In addition, the peak due to the pyridinic N is diminished to the maximum extent because of KOH activation. In Fig. 1(a), the pyridinic N peak is significantly diminished from 600 °C to 750 °C; however, under pyrolytic conditions, there is no significant removal of the pyridinic N until the temperature reaches 900 °C. These results confirm the KOH's oxidization effect. As observed in the both figures, the quaternary N finally becomes the most stable N species at very high temperature.
The conclusions from the XPS results were confirmed via FTIR. As shown in Fig. 2, the peak at 3420 cm−1 corresponds to the N–H stretching vibration, but it overlaps with O–H stretching vibration, making identification difficult. The weak peak at 1720 cm−1 corresponds to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration and becomes weaker with increasing activation temperature, demonstrating the gradual disappearance of C
O stretching vibration and becomes weaker with increasing activation temperature, demonstrating the gradual disappearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The bands at 1400–1610 cm−1 are ascribed to the existence of ring structures that contain N. The band at 1250 cm−1 is attributed to the C–N stretching vibration. These bands decrease with increasing temperature, which reveals the removal of N. The information mentioned above points to the removal of N-containing groups.
O. The bands at 1400–1610 cm−1 are ascribed to the existence of ring structures that contain N. The band at 1250 cm−1 is attributed to the C–N stretching vibration. These bands decrease with increasing temperature, which reveals the removal of N. The information mentioned above points to the removal of N-containing groups.
3.2 Pore structures of the synthesized carbons
Fig. 3 shows the typical SEM images of the samples. Sample NFPC-600 exhibits a lamellar structure with fewer pores and offers a large interfacial area for the activation reaction with KOH. This morphology was well preserved through activation at 750 °C. Fig 3(b) shows that more pores appeared at 750 °C, and the foam-like structure began to emerge, which represents intense KOH activation. Some of the pores are interconnected, indicative of over-activation; this phenomenon becomes more pronounced in Fig 3(c). Over-activation diminishes the specific surface area, which is confirmed in the nitrogen adsorption/desorption isotherms shown in Fig. 4. Compared with NFPC-750, NFPC-750-NA still shows a lamellar structure but much fewer pores (Fig. 3(d)) because of no KOH activation. The only pores in NFPC-750-NA can be attributed to hydroxyapatite that is washed away. | ||
| Fig. 4 (a) N2 adsorption/desorption isotherms of NFPC-Ts and (b) PSDs of NFPC-Ts; (c) N2 adsorption/desorption isotherms of NFPC-T-NAs and (d) PSDs of NFPC-T-NAs. | ||
The nitrogen adsorption/desorption isotherms and corresponding PSDs (pore-size distributions) of major samples are illustrated in Fig. 4. The major structural property data for these samples are displayed in Table 2. With increasing temperature, the specific surface area and total pore volume of the NFPC-T-NAs increase, but the micropore volume remains constant at 0.06 cm3 g−1. This result shows that only mesopores and macropores are formed during carbonation. No apparent hysteresis loop occurs in the curves for NFPC-600-NA. This result suggests that macropores are the main pore type existing in NFPC-600-NA and that these pores were formed by washed hydroxyapatite. At a temperature of 900 °C, a mesopore hysteresis loop begins to appear. Interestingly, the pyridinic N decreases substantially when the temperature is increased from 750 °C to 900 °C, suggesting that pyridinic N removal is correlated to the formation of mesopores. In contrast, the specific surface area of the NFPC-Ts shows a peak value of to 3206 m2 g−1 at the activation temperature of 750 °C. At this point, the micropore volume is 0.76 cm3 g−1, which is much larger than the micropore volume of NFPC-750-NA, implying that KOH activation forms micropores. NFPC-900 has a lower micropore volume and specific surface area because of over-activation. It has been reported that micropores can be of benefit to CO2 capture;23 therefore, NFPC-750 would have a large advantage in CO2 capture.
| Samples | Structural properties | |||
|---|---|---|---|---|
| SBETa (m2 g−1) | Vpb (cm3 g−1) | Vmicroc (cm3 g−1) | Dpd (nm) | |
| a SBET: specific surface area.b Vp: total pore volume.c Vmicro: micropore volume.d Dp: average pore diameter. | ||||
| NFPC-600 | 1479 | 0.88 | 0.54 | 2.4 |
| NFPC-750 | 3206 | 2.29 | 0.76 | 2.9 |
| NFPC-900 | 2712 | 2.73 | 0.66 | 4.0 |
| NFPC-600-NA | 317 | 0.19 | 0.06 | 2.4 |
| NFPC-750-NA | 586 | 0.40 | 0.06 | 2.7 |
| NFPC-900-NA | 731 | 1.03 | 0.06 | 5.6 |
3.3 Carbon dioxide capture capacity
Table 3 displays the summary of the CO2 capture capacities of the samples. Each adsorbent shows higher CO2 capture capacity at 25 °C than that at 50 °C, which suggests that these materials tend to adsorb CO2 at low temperature. In addition, the NFPC-Ts exhibit higher CO2 capture capacity than NFPC-T-NAs, which can be attributed to their favourable pore structure, especially the large micropore volume and specific surface area; however, this advantageous pore structure is obtained at the cost of the nitrogen content. Moreover, the capture capacity of the NFPC-T-NAs decreases with increasing temperature. It can be concluded that if the material lacks micropores, N content would be a more dominant factor influencing the capture capacity. NFPC-900 shows a higher capacity at 25 °C than NFPC-600, but a lower capacity at 50 °C. This result demonstrates that N content contributes more at higher temperature. Among all the samples, NFPC-750 exhibits the highest CO2 capture capacity (171 mg g−1) at 25 °C, because NFPC-750 has the highest specific surface area and micropore volume among all the synthesized carbons, and the quaternary N is preserved in this sample, owing to the proper activation temperature. This material shows a capacity of 107 mg g−1 at 50 °C, also the highest capacity at this temperature.| T (°C) | Capacity at 25 °C (mg g−1) | Capacity at 50 °C (mg g−1) | ||
|---|---|---|---|---|
| NFPC-T | NFPC-T-NA | NFPC-T | NFPC-T-NA | |
| a The adsorptions were all under 1 atm. | ||||
| 600 | 123 | 81 | 104 | 58 |
| 750 | 171 | 74 | 107 | 45 |
| 900 | 150 | 56 | 93 | 43 |
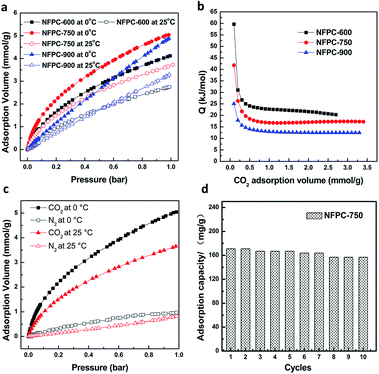
To further investigate the interaction strength between CO2 and NFPC-Ts, the isosteric heat of adsorption (Q) is calculated from CO2 adsorption isotherms (Fig. 5(a)) measured at two temperatures (0 °C and 25 °C) by means of the Clausius–Clapeyron equation: (T2 − T1) × Q/(RT1T2) = ln(p2/p1), where T2 and T1 are 0 °C and 25 °C, respectively; p2 and p1 are pressure values of CO2; and R is the universal gas constant. The result (Fig. 5(b)) shows that the initial isosteric heat of adsorption of NFPC-600 is about 59 kJ mol−1, which is higher than that of NFPC-750 (42 kJ mol−1) and much higher than that of NFPC-900 (25 kJ mol−1). High Q values indicate strong interaction between CO2 and the material. Given that the N contents of the NFPC-Ts also decrease as T increases, the reduction of the interaction between CO2 and NFPC-Ts corresponds to the removal of nitrogen groups; therefore, the N groups of NFPC-Ts play key roles in CO2 adsorption. The high value of the isosteric heat of adsorption of NFPC-750 (42 kJ mol−1) demonstrates that its N groups are strong enough to act as basic sites. The Q value obviously decreases with the increase in CO2 adsorption volume, which suggests that the interaction becomes weaker with basic sites.19,24,25 The Q value of NFPC-750 finally remains stable at about 17 kJ mol−1 which means there is a high affinity of this adsorbent for CO2 even at high relative pressure.
Except for high capacity, adsorption selectivity between CO2 and N2 is also important for the sorbent. We examined the CO2/N2 selectivity of NFPC-750 at 25 °C and 0 °C (Fig. 5(c)) and found that the volume of adsorbed N2 is much lower than that of adsorbed CO2. At 1 bar and 25 °C, the volume of adsorbed N2 (0.81 mmol g−1) is 21.7% of adsorbed CO2 (3.73 mmol g−1). At 1 bar and 0 °C, the volume of adsorbed N2 increases to 0.97 mmol g−1, which is 19.2% of adsorbed CO2 (5.04 mmol g−1). This indicates that NFPC-750 may perform better at lower temperature for CO2 capture. The high CO2/N2 selectivity (at the ratio of 5.21 at 0 °C) suggests that NFPC-750 can be a selective sorbent for CO2 over N2.
The regeneration capacity of NFPC-750 was investigated via cycling adsorption and desorption. It was found that NFPC-750 showed good regeneration performance (Fig. 5(d)). The CO2 capture capacity is retained up to 157 mg g−1 after 10 cycles, which is about 91.8% of the initial capacity. The good regeneration performance of NFPC-750 makes it a promising sorbent that can be reused.
4. Conclusion
In summary, we have demonstrated a strategy that uses fish scales to produce highly porous N-doped carbon for CO2 capture. Upon optimization of the pore structure and N content (up to 2.90%), the CO2 capture capacity of the carbon sorbent can be 171 mg g−1 at 25 °C. The N species on carbon could be tuned by varying the thermal treatment temperature and KOH activation conditions. Quaternary N, but not pyridonic N or pyridinic N, showed higher stability during KOH activation. Interestingly, the peak values of specific surface area (up to 3206 m2 g−1) and micropore volume (up to 0.760 cm3 g−1) were obtained with KOH activation at 750 °C; moreover, quaternary N species remained unchanged. In addition, NFPC-750 showed high affinity for CO2 (isosteric heat of adsorption is 17 kJ mol−1), high selectivity for CO2 over N2 (at the ratio of 5.21 at 0 °C), and good regeneration performance (up to 91.8% of the initial capacity after 10 cycles). All these outstanding characteristics indicate that fish scales can be a promising raw material for producing efficient CO2 sorbent materials.Acknowledgements
This study is financially supported by the National Natural Science Foundation of China (No. 51272017 and 51432003).References
- Y. Y. Li, K. K. Han, W. G. Lin, M. M. Wan, Y. Wang and J. H. Zhu, J. Mater. Chem. A, 2013, 1, 12919 CAS.
- M. B. Yue, L. B. Sun, Y. Cao, Y. Wang, Z. L. Wang and J. H. Zhu, Chem.–Eur. J., 2008, 29, 1051 CAS.
- G. P. Hao, W. C. Li, D. Qian and A. H. Lu, Adv. Mater., 2010, 22, 853 CrossRef CAS PubMed.
- J. C. Wang and Q. Liu, Nanoscale, 2014, 6, 4148 RSC.
- K. K. Han, Y. Zhou, Y. Chun and J. H. Zhu, J. Hazard. Mater., 2012, 203, 341 CrossRef PubMed.
- J. J. Wen, F. N. Gu, F. Wei, Y. Zhou, W. G. Lin, J. Yang, J. Y. Yang, Y. Wang, Z. G. Zou and J. H. Zhu, J. Mater. Chem. A, 2010, 20, 2840 RSC.
- D. Saha and S. G. Deng, J. Colloid Interface Sci., 2010, 345, 402 CrossRef CAS PubMed.
- M. L. Yao, L. Wang, X. Hu, G. S. Hu, M. F. Luo and M. H. Fan, J. Mater. Sci., 2015, 50, 1221 CrossRef CAS.
- J. K. Sun and Q. Xu, Energy Environ. Sci., 2014, 7, 2071 CAS.
- Y. Xia, Z. Yang and R. Mokaya, Nanoscale, 2010, 2, 639 RSC.
- L. Zhao, Z. Bacsik, N. Hedin, W. Wei, Y. Sun and M. Antonietti, et al., ChemSusChem, 2010, 3, 840 CrossRef CAS PubMed.
- Y. F. Chen and J. W. Jiang, ChemSusChem, 2010, 3, 982 CrossRef CAS PubMed.
- M. Sevilla, J. B. Parra and A. B. Fuertes, ACS Appl. Mater. Interfaces, 2013, 5, 6360 CAS.
- Y. F. Guo, C. W. Zhao, C. H. Li and S. X. Lu, Appl. Energy, 2014, 129, 17 CrossRef CAS PubMed.
- F. S. Su, C. Lu, A. J. Chung and C. H. Liao, Appl. Energy, 2014, 113, 706 CrossRef CAS PubMed.
- H. W. Yang, Y. Z. Yuan and S. C. E. Tsang, Chem. Eng. J., 2012, 185, 374 CrossRef PubMed.
- C. Pevida, T. C. Drage and C. E. Snape, Carbon, 2008, 46, 1464 CrossRef CAS PubMed.
- J. A. Thote, K. S. Iyer, R. Chatti, N. K. Labhsetwar, R. B. Biniwale and S. S. Rayalu, Carbon, 2010, 48, 396 CrossRef CAS PubMed.
- Z. Liu, Z. Y. Du, W. Xing and Z. F. Yan, Mater. Lett., 2014, 117, 273 CrossRef CAS PubMed.
- M. Sevilla, P. Valle-Vigón and A. B. Fuertes, Adv. Funct. Mater., 2011, 21, 2781 CrossRef CAS PubMed.
- F. H. Bai, Y. D. Xia, B. L. Chen, H. Q. Su and Y. Q. Zhu, Carbon, 2014, 79, 213 CrossRef CAS PubMed.
- W. X. Chen, H. Zhang, Y. Q. Huang and W. K. Wang, J. Mater. Chem., 2010, 20, 4773 RSC.
- J. K. Pels, F. Kapteijn, J. A. Moulijn, Q. Zhu and K. M. Thomas, Carbon, 1995, 33, 1641 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Energy Environ. Sci., 2011, 4, 1765 CAS.
- J. Wang, I. Senkovska, M. Oschatz, M. R. Lohe, L. Borchardt and A. Heerwig, et al., J. Mater. Chem. A, 2013, 1, 10951 CAS.
| This journal is © The Royal Society of Chemistry 2015 |