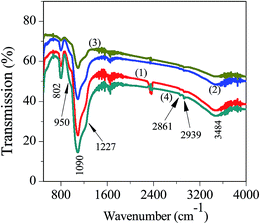
A soft-template mediated approach for Au(0) formation on a heterosilica surface and synergism in the catalytic reduction of 4-nitrophenol†
Prateeksha Mahamallik and
Anjali Pal*
Department of Civil Engineering, Indian Institute of Technology Kharagpur, Kharagpur 721302, India. E-mail: anjalipal@civil.iitkgp.ernet.in; Fax: +91-3222-282254; Tel: +91-3222-281920
First published on 3rd September 2015
Abstract
Under suitably controlled conditions a cationic surfactant e.g. cetyltrimethylammonium bromide (CTAB) forms bilayers on a silica surface, which has the unique property of solubilizing organic molecules through ‘adsolubilization’. This property is judiciously exploited to adsorb AuCl4− ions on the modified silica surface at much higher loading (compared to normal silica) through the formation of a CTA+ AuCl4− complex on the silica support. Next, gold nanoparticles supported on the surfactant-modified silica (SMS) are prepared using two methods: (1) UV illumination and (2) borohydride reduction, and the materials are designated as SMSG-1 and SMSG-2. Characterization of the particles confirms the formation of Au(0) nanoparticles on the SMS surface. The particles have a spherical shape with an average size of 37 ± 11 and 54 ± 14 nm for SMSG-1 and SMSG-2, respectively. They exhibit much higher catalytic activity when compared to Au(0) supported on normal silica for 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) reduction in a borohydride medium. Such synergism is presumed to be due to the surfactant bilayer, which provides a high concentration of 4-nitrophenolate ions near the Au(0) nanoparticles embedded on the SMS leading to highly efficient contact between them. The reductions under different conditions are studied and compared. SMSG-1 shows better performance when compared to SMSG-2. While the reaction followed a pseudo-first order for SMSG-1 for all experimentally studied conditions, a clear change over in order from first to second was noticed for SMSG-2 under certain conditions. The reaction rates, recyclability and turn over frequency (TOF) with a growing micro-electrode (GME) and a fully grown micro-electrode (FGME) are compared. The GME shows ∼9 times higher catalytic activity as compared to the FGME.
1. Introduction
The idea of sustainability in research has prompted many scientists to prepare catalyst materials with a high potential, which can carry out reactions efficiently and selectively. In the recent past, metallic nanoparticles have gained tremendous attention because of their potential use in catalysis. In particular nanoparticles on a solid support have a certain practical benefit. Gold was considered to be chemically inactive till Hutching’s1 and Haruta’s2,3 discoveries that gold can be used as a catalyst for acetylene hydrochlorination and CO oxidation. One major reason why gold catalysis did not get attention for many years is that in most cases the supported gold nanoparticles prepared by the traditional incipient-wetness impregnation method led to nanoparticles with a size which was higher than the critical size range thus making them inactive.4 To overcome the problem coprecipitation and deposition–precipitation methods were adopted to prepare nanoparticles on an oxide support. The catalytic activity of gold depends upon the particle size, oxidation state of gold and the nature of the supporting materials. The surface of the supporting material acts as nucleating agents and it has the role of stabilizing the nanoparticle which finally may have an enormous impact on the catalytic activity.5,6Recently heterogeneous gold nanocatalyst materials showed huge demand due to their selective and enhanced catalytic properties.7 Many researchers have prepared gold nanoparticles on a solid support, such as a polymer, alumina and silica to enhance the reactivity.8 Gold nanoparticle arrays were prepared on a poly(N-dodecyl acrylamide-co-4-vinyl pyridine) template.9 Scientists have reported gold catalyst preparation on an alumina surface by a direct anionic exchange (DAE) method.10 The preparation of gold nanoparticles on an anion exchange resin has also been reported.11,12 Titania doped silica was prepared using a sol–gel method and the same material was used for depositing gold onto it.13 Gold and silver nanoparticles in either monometallic or bimetallic forms on calcium alginate beads were prepared using a photochemical method.14,15 Spherical polyelectrolyte brushes were used as a template for platinum and gold nanoparticle synthesis.16 Gold nanoparticles were prepared on ZnO-ceramic microstructured paper.17 Another report discussed gold nanoparticle formation on a γ-Al2O3 support, where plant tannins were the stabilizer.18 Stabilized gold nanoparticles were also prepared on ∼15 nm thin porous alumina sheets.19 In our work, we have reported the preparation and catalytic application of gold nanoparticles supported on surfactant-modified silica (SMS). Under the specified conditions, CTAB molecules get adsorbed on the silica surface to form a bilayer structure (Scheme 1), which has the capability to ‘adsolubilize’ various organic molecules at a much higher concentration.20 The preparation of the catalyst material is very simple. Silica, which is used for this material is very cheap. As it is in solid form, the catalyst is very easy to separate from the product.
Nitro aromatic compounds are the most common organic pollutants in industrial and agricultural wastewater. In particular nitrophenols (NPs) are common contaminants in industrial effluent as they are used in manufacturing dyes, explosives and pesticides. 4-Aminophenol (4-AP) is the reduction product of 4-nitrophenol (4-NP). It has immense application as a photographic developer, corrosion inhibitor, dying agent, and building block for the production of analgesic and antipyretic drugs. Moreover, in the recent past, nanoparticle catalyzed 4-NP reduction by borohydride has received considerable attention because of its simplicity and reproducibility.8,21 The most attractive feature of this benchmark reaction is that it can be monitored using a simple UV-visible spectrophotometer. In our present work, 4-NP reduction by the prepared materials has been studied. It was the focus of the present work to investigate the effect of the surfactant bilayer formed on the silica surface, towards Au(0) formation and 4-NP reduction by borohydride.
2. Materials and methods
2.1. Chemicals
Sodium borohydride and CTAB were obtained from SRL. Gold chloride (HAuCl4) was purchased from Aldrich. 4-NP was obtained from LOBA chemicals. It was crystallized from alcohol before use. A stock solution of 4-NP (1 × 10−2 M) was prepared in double distilled water. Silica gel 60–120 mesh size (250–150 micron; used for column chromatography) was purchased from Merck. Orange II dye was procured from HIMEDIA.2.2. Instrumentation
A UV-visible spectrophotometer (UV 1, Thermospectronic, England) was used to measure the absorbance with a 1 cm well stoppered quartz cuvette. Fourier transform infrared (FTIR) spectra were obtained using a Perkin-Elmer FTIR instrument (RX1). Field Emission Scanning Electron Microscopy (FESEM) was carried out using a microscope (Supra 40, Carl Zeiss Pvt. Ltd, Germany). Transmission electron microscopy (TEM) images were collected by spotting the sonicated sample on a copper grid in a FEI-TECNAI G2 20S-TWIN (USA) instrument with an operating voltage of 200 kV. X-ray diffraction (XRD) analysis of the prepared materials was performed by a Bruker axs, SMART APEX II instrument using Cu as the X-ray source with Kα = 1.54056.2.3. Methods
2.4. Preparation of catalyst material
2.5. Experimental for 4-NP reduction
The 4-NP reduction was carried out in a well stoppered quartz cuvette in excess borohydride medium. In a typical set up, a mixture of 2.5 mL of BH4− and 25 μL of 4-NP (stock solution) was taken and put into the cuvette and an appropriate amount of catalyst was added to it. The reaction was allowed to continue under ambient conditions. Stirring was not required because of the liberation of hydrogen gas due to the presence of borohydride. The catalyst was in a fluidized condition in the solution due to continuous evolution of bubbles, which favored the reaction. The concentration used for NaBH4 was 0.1 M in all conditions. The reaction was monitored at 400 nm at different time intervals in a UV-visible spectrophotometer.3. Results and discussions
3.1. Formation of gold nanoparticles on SMS surface
The SMS preparation is interesting and it has enormous applications in pollutant removal through the well-known ‘adsolubilization’ mechanism.20,23 The point of zero charge (pHPZC) of silica is 1.7.24 Hence at neutral pH its surface is negatively charged. CTAB molecules dissociate into CTA+ and Br− in water, and when silica is added to CTAB, the CTA+ ions get attached to the silica surface due to electrostatic interactions, forming a monolayer. It is well established that under certain conditions a second layer of the surfactant molecules get attached to the first layer through hydrophobic interactions, and finally the positive charge of the CTA+ molecules in the second layer remains exposed towards the aqueous phase. This type of phenomenon was described earlier through adsorption isotherm studies.20,23,25 It is to be noted that the conditions that we applied here for the SMS preparation favor the bilayer formation on the silica surface (Scheme 1).23After this step, AuCl4− ions are adsorbed on the SMS surface (which is a rapid process) and finally reduced to Au(0). The SMS material shows much higher (∼2.3 times) adsorption capacity for AuCl4− when compared to normal silica, as has been described in Section 2.3.2. It reveals that CTAB plays an important role for adsorption of HAuCl4 on the SMS. The loading of AuCl4− was indicated by its yellow color which is due to metal-to-ligand charge transfer in AuCl4−.15 The material was very stable (over 6 months) and was designated as Au(III)-SMS. As gold is the actual catalyst, a higher loading of gold in Au(III)-SMS is expected to cause better catalytic efficiency when compared to the normal silica material.
The final step of the catalyst preparation is the reduction of Au(III) to Au(0) and that has been accomplished by two different methods viz., photochemical and chemical. Although both of the routes are available for reduction purposes, photochemical processes are less common. At the same time, while chemical processes suffer from drastic conditions, photochemical processes are more “green” and mild. In the chemical process we have used the most renowned reagent i.e. sodium borohydride. There are various types of photoinitiators (viz., benzophenone, polyvinylpyrrolidone, polyvinyl alcohol, dendrimers, methanol, benzoin, formic acid, EDTA, Triton X-100, ascorbic acid, alginates etc.) used for reducing gold ions, however in the present case no such initiators are applied for such purpose. The photoirradiation of Au(III)-SMS was done under UV light (∼365 nm; 15 W). The material was kept soaked with water during the irradiation time (20 min). The dark navy blue and violet color for the photochemically produced (SMSG-1) and chemically produced (SMSG-2) materials, respectively, were characterized and finally used as the catalysts.
In a similar way AuCl4− was also adsorbed on the normal silica surface and was finally reduced to Au(0) by the same two methods. It is interesting to observe that the colors of the two materials SG-1 and SG-2 prepared by the photochemical and chemical method respectively are much less intense. This is due to lower gold loading as was described in Section 2.3.2.
The whole system acts as a soft template for 4-NP reduction (Scheme 2).
3.2. Characterization of the materials SMSG-1 and SMSG-2
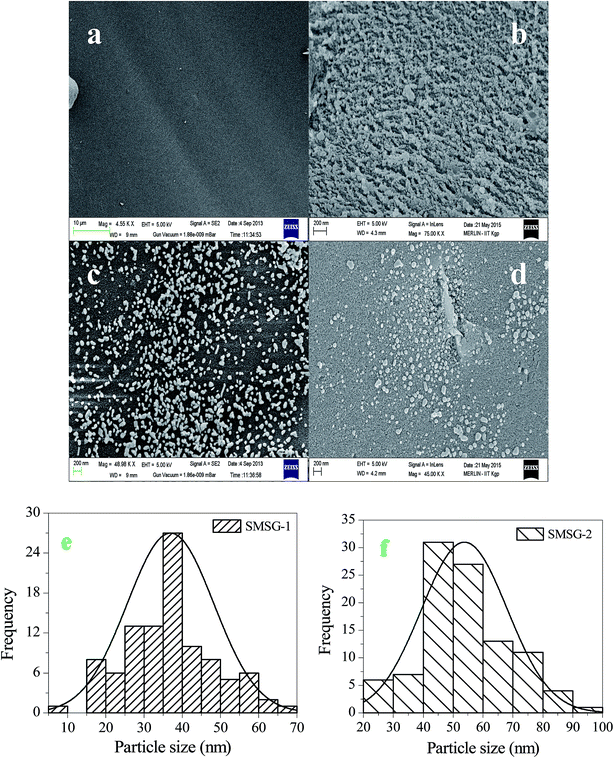 | ||
| Fig. 2 SEM images of (a) SMS, (b) Au(III)-SMS, (c) SMSG-1, (d) SMSG-2. (e) and (f) shows the size distribution of the Au(0) particles in SMSG-1 and SMSG-2, respectively. | ||
To know the nature of the surface, SEM analyses were performed on SG-1 and SG-2. The images are shown in Fig. S1a and b,† respectively. Here unlike SMSG-1 and SMSG-2, the gold particles are not observed distinctly on the surface, although sufficient loading (3.06 × 10−5 moles per gram of silica) of AuCl4− was observed through the mass balance method. This is possible because in these cases the AuCl4− ions have diffused through the silica matrix. In the case of the SMS, due to the presence of the surfactant bilayers on the surface, the AuCl4− ions attach themselves to the surface. Finally, the AuCl4− ions are reduced under different conditions.
3.3. Catalytic reduction of 4-nitrophenol
The activity of the as-prepared catalysts was demonstrated through 4-NP reduction in the presence of borohydride as a reductant. In the last decades this reaction using noble metal nanoparticles as the catalyst has become one of the model reactions to evaluate the catalytic activity of various metal nanoparticles viz., Au, Ag, Pd, Pt, Cu etc. present in different substrates. This reaction is thermodynamically favorable with E0 = −0.76 V for 4-NP/4-AP and −1.33 V vs. NHE for H3BO3/BH4−, but it is kinetically very slow under uncatalyzed conditions.4-NP shows λmax at 317 nm (curve 1, Fig. 4a). However, upon addition of NaBH4 the peak shifts to 400 nm (curve 2, Fig. 4a) due to the formation of 4-nitrophenolate anions. An aliquot of 4-NP of concentration ∼1.0 × 10−4 M was prepared by diluting the stock (10−2 M) in borohydride medium (0.1 M), and it was allowed to remain as such for 24 h and the peak at 400 nm was monitored from time to time. In this case neither a decrease in absorbance at 400 nm nor a new peak is observed. In the presence of the SMS and borohydride, however, a slight decrease in absorbance was noticed after 20 min (curve 3, Fig. 4a). This slight decrease in the absorption peak is due to the adsorption of nitrophenolate ions on the SMS. But still no new peak related to its reduced product 4-AP was observed at ∼293 nm (Fig. 4a).
On the contrary, the catalytic activity of SMSG-1 is demonstrated convincingly in Fig. 4b by the spectrophotometric studies. Curve 1 in Fig. 4b depicts the absorption spectrum of 4-NP in the absence of borohydride. The shift of the peak at 400 nm in the presence of excess borohydride is due to the 4-nitrophenolate anions. After the addition of the catalyst SMSG-1, immediately the absorbance at 400 nm went on decreasing with time and a new peak of 4-AP as a product was observed to appear at 293 nm. The sequential decrease in intensity of the 400 nm peak is shown in Fig. 4b. This could also be visualized by eye as the yellow color of the nitrophenolate ion faded and disappeared quickly. In the present study some isobestic points are clearly observed which implies that 4-AP is the sole product during the reaction.31 Some isobestic points are not so prominent because of the hindrance in monitoring due to the liberation of gas bubbles.32
In is interesting to note that the reduction of 4-NP under similar conditions is very fast in the presence of the Au(III)-SMS (curve 4, Fig. 4a). The reason behind this is discussed more elaborately in Section 3.3.2.
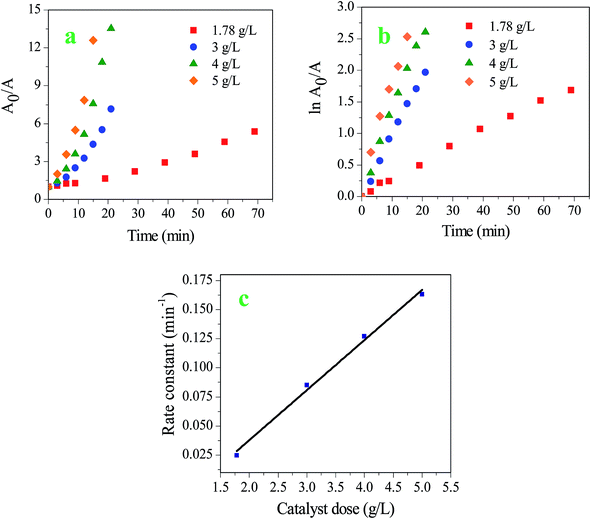
The same reaction of 4-NP reduction was carried out with the other three prepared catalysts i.e. SG-1, SG-2, and SMSG-2. The rate of the reactions with SG-1 and SG-2 are very slow as compared to SMSG-1 and SMSG-2 for the same initial concentration (1.0 × 10−4 M) of 4-NP and catalyst dose (4.0 g L−1). The pseudo-first order reaction rate constant of the reaction is 0.001 min−1 for SG-1 and SG-2, whereas those with SMSG-1 and SMSG-2 are 0.127 and 0.073 min−1, respectively (Fig. 5).
It is well accepted that the actual catalyst is the gold nanoparticle embedded in the SMS surface. It is already mentioned that the gold loading is ∼2.3 times higher in SMSG when compared to that of SG. Hence one factor for the enhanced catalysis in SMSG might be the enhanced loading of gold in the SMS as compared to silica. However, to account for an enormously enhanced rate (∼127 times for SMSG-1 and ∼73 times for SMSG-2 as compared to SG) of 4-NP reduction, there must be some other factors operating. Such an enormous increase in rate is possibly due to the facilitated adsorption (or more appropriately adsolubilization) of 4-NP on the surfactant bilayer formed on the SMSG surface. The high activity arises from the synergistic effect of the CTA+ bilayer, which provides a high concentration of 4-nitrophenolate ions near to the Au nanoparticles embedded on the SMS leading to highly efficient contact between them.
To investigate the effects of the adsorbed surfactant bilayer and the embedded gold, detailed kinetic studies were conducted with SMSG-1 and SMSG-2.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A0/A vs. time at 300 K are shown in Fig. 6a and b, respectively. It is clear from the linear plots of ln
A0/A vs. time at 300 K are shown in Fig. 6a and b, respectively. It is clear from the linear plots of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A0/A vs. time that the reaction follows pseudo-first order kinetics in all cases. The plot of first order rate constants vs. catalyst doses shows a good linear correlation (Fig. 6c; R2 = 0.994). The rate of reaction becomes faster with an increase in dose (Table 1). It indicates that the reaction is pseudo-first order in the entire range of doses applied. Interestingly, a change in order of kinetics from first order to second order was observed for SMSG-2 while the dose of catalyst is decreased from 5.0 to 1.78 g L−1. Keeping the 4-NP concentration fixed at 1.0 × 10−4 M, for doses 1.78, 3.0, and 3.5 g L−1 the kinetics follows second order, whereas, for doses 4.0 and 5.0 g L−1 it is noticed to be first order (Fig. S3† and Table 1). A similar phenomenon has been observed with a fixed catalyst dose but varying the initial concentration of 4-NP. For example, at a fixed catalyst dose of 4.0 g L−1, the reduction with 0.4 × 10−4 M and 0.6 × 10−4 M 4-NP follows second order but the reaction follows first order with a concentration of 1.0 × 10−4 M and 1.2 × 10−4 M (Fig. S3† and Table 1). On the other hand, unlike SMSG-2, in the case of SMSG-1 the rate follows first order even with variation of the initial 4-NP concentration in the range 0.4 × 10−4 to 1.2 × 10−4 M and keeping the catalyst dose fixed at 4.0 g L−1. The rate constant is found to decrease drastically from 0.253 to 0.086 min−1 as the 4-NP concentration is increased from 0.4 × 10−4 M to 1.2 × 10−4 M (Table 1). The plot of rate constant vs. concentration of 4-NP follows a straight line curve with R2 = 0.969 (Fig. 7). The comparative account of the first- and second-order rate constants and their correlation coefficient values for all of the concentrations of 4-NP and all doses of SMSG-1 and SMSG-2 are compiled in Table 1. From the results it is obvious that SMSG-1 is a better catalyst when compared to SMSG-2 for 4-NP reduction. The rate constant of the reactions could be explained well by Langmuir–Hinshelwood kinetics.35,36 The assumption of this mechanism is that the reactants should get adsorbed on the surface of the catalyst. The reaction of the adsorbed species determines the rate. In many cases an induction time was observed for the metal nanoparticle induced 4-NP reduction. It designates the surface restructuring of the nanoparticle before starting the reaction.31 If the rate of reaction between the dissolved oxygen present in the water and borohydride is faster than that with nitrophenol, then also an induction time may be observed.14 In the present study, however, no induction time is noticed. It is similar to the results presented earlier.14 As soon as the catalyst is added to the reactant, the reaction starts. The borohydride ions transfer hydride species onto the surface of the nanoparticle. This reaction is reversible. Nitrophenol molecules are adsorbed on the surface of the nanoparticle and are reduced by the surface hydride species.16 This adsorption step is also reversible. However, an adsorption–desorption equilibrium is reached very quickly. The positive charge on the SMSG surface creates favorable conditions for the adsorption of both hydride ions and nitrophenolate ions on the surface. This makes the reaction faster. This is clear from the synergism that we observe when SMSG is used as the catalyst. After the reaction, the product molecules get desorbed from the surface making the reaction sites free for further reactions. Overall conversion of 4-NP to 4-AP is a 6 electron transfer process. According to Gu et al.31 the production of 4-AP from 4-NP is completed in two steps. In the first step an intermediate, 4-hydroxylaminophenol (4-HAP), a stable product, is formed from 4-NP. Then it gets reduced to the final product, 4-AP. The first step is much faster as compared to the second one. The availability of the active surface on the nanoparticle decreases as the reaction proceeds, because, during the reduction of 4-NP, the concentration of 4-HAP increases with time and it contests with 4-NP for the nanoparticle surface.
A0/A vs. time that the reaction follows pseudo-first order kinetics in all cases. The plot of first order rate constants vs. catalyst doses shows a good linear correlation (Fig. 6c; R2 = 0.994). The rate of reaction becomes faster with an increase in dose (Table 1). It indicates that the reaction is pseudo-first order in the entire range of doses applied. Interestingly, a change in order of kinetics from first order to second order was observed for SMSG-2 while the dose of catalyst is decreased from 5.0 to 1.78 g L−1. Keeping the 4-NP concentration fixed at 1.0 × 10−4 M, for doses 1.78, 3.0, and 3.5 g L−1 the kinetics follows second order, whereas, for doses 4.0 and 5.0 g L−1 it is noticed to be first order (Fig. S3† and Table 1). A similar phenomenon has been observed with a fixed catalyst dose but varying the initial concentration of 4-NP. For example, at a fixed catalyst dose of 4.0 g L−1, the reduction with 0.4 × 10−4 M and 0.6 × 10−4 M 4-NP follows second order but the reaction follows first order with a concentration of 1.0 × 10−4 M and 1.2 × 10−4 M (Fig. S3† and Table 1). On the other hand, unlike SMSG-2, in the case of SMSG-1 the rate follows first order even with variation of the initial 4-NP concentration in the range 0.4 × 10−4 to 1.2 × 10−4 M and keeping the catalyst dose fixed at 4.0 g L−1. The rate constant is found to decrease drastically from 0.253 to 0.086 min−1 as the 4-NP concentration is increased from 0.4 × 10−4 M to 1.2 × 10−4 M (Table 1). The plot of rate constant vs. concentration of 4-NP follows a straight line curve with R2 = 0.969 (Fig. 7). The comparative account of the first- and second-order rate constants and their correlation coefficient values for all of the concentrations of 4-NP and all doses of SMSG-1 and SMSG-2 are compiled in Table 1. From the results it is obvious that SMSG-1 is a better catalyst when compared to SMSG-2 for 4-NP reduction. The rate constant of the reactions could be explained well by Langmuir–Hinshelwood kinetics.35,36 The assumption of this mechanism is that the reactants should get adsorbed on the surface of the catalyst. The reaction of the adsorbed species determines the rate. In many cases an induction time was observed for the metal nanoparticle induced 4-NP reduction. It designates the surface restructuring of the nanoparticle before starting the reaction.31 If the rate of reaction between the dissolved oxygen present in the water and borohydride is faster than that with nitrophenol, then also an induction time may be observed.14 In the present study, however, no induction time is noticed. It is similar to the results presented earlier.14 As soon as the catalyst is added to the reactant, the reaction starts. The borohydride ions transfer hydride species onto the surface of the nanoparticle. This reaction is reversible. Nitrophenol molecules are adsorbed on the surface of the nanoparticle and are reduced by the surface hydride species.16 This adsorption step is also reversible. However, an adsorption–desorption equilibrium is reached very quickly. The positive charge on the SMSG surface creates favorable conditions for the adsorption of both hydride ions and nitrophenolate ions on the surface. This makes the reaction faster. This is clear from the synergism that we observe when SMSG is used as the catalyst. After the reaction, the product molecules get desorbed from the surface making the reaction sites free for further reactions. Overall conversion of 4-NP to 4-AP is a 6 electron transfer process. According to Gu et al.31 the production of 4-AP from 4-NP is completed in two steps. In the first step an intermediate, 4-hydroxylaminophenol (4-HAP), a stable product, is formed from 4-NP. Then it gets reduced to the final product, 4-AP. The first step is much faster as compared to the second one. The availability of the active surface on the nanoparticle decreases as the reaction proceeds, because, during the reduction of 4-NP, the concentration of 4-HAP increases with time and it contests with 4-NP for the nanoparticle surface.
| Material | Dose of catalyst (g L−1) | Initial conc. of 4-NP (M) | First order | Second order | Order of reaction followed | ||
|---|---|---|---|---|---|---|---|
| Rate constant min−1 | R2 | Rate constant mg−1 L min−1 | R2 | ||||
| SMSG-1 | 1.78 | 1.0 × 10−4 | 0.0248 | 0.996 | 0.034 | 0.974 | First |
| 3 | 1.0 × 10−4 | 0.085 | 0.997 | 0.152 | 0.948 | First | |
| 4 | 1.0 × 10−4 | 0.127 | 0.993 | 0.289 | 0.931 | First | |
| 5 | 1.0 × 10−4 | 0.163 | 0.985 | 2.444 | 0.596 | First | |
| SMSG-1 | 4.0 | 0.4 × 10−4 | 0.253 | 0.967 | 1.186 | 0.966 | First |
| 4.0 | 0.6 × 10−4 | 0.184 | 0.978 | 0.743 | 0.953 | First | |
| 4.0 | 1.0 × 10−4 | 0.127 | 0.993 | 0.289 | 0.931 | First | |
| 4.0 | 1.2 × 10−4 | 0.086 | 0.987 | 0.170 | 0.955 | First | |
| SMSG-2 | 1.78 | 1.0 × 10−4 | 0.014 | 0.968 | 0.013 | 0.996 | Second |
| 3 | 1.0 × 10−4 | 0.022 | 0.979 | 0.021 | 0.998 | Second | |
| 3.5 | 1.0 × 10−4 | 0.026 | 0.961 | 0.038 | 0.998 | Second | |
| 4 | 1.0 × 10−4 | 0.073 | 0.988 | 0.141 | 0.967 | First | |
| 5 | 1.0 × 10−4 | 0.085 | 0.995 | 0.130 | 0.967 | First | |
| SMSG-2 | 4.0 | 0.4 × 10−4 | 0.033 | 0.954 | 0.075 | 0.983 | Second |
| 4.0 | 0.6 × 10−4 | 0.028 | 0.961 | 0.071 | 0.987 | Second | |
| 4.0 | 1.0 × 10−4 | 0.073 | 0.988 | 0.141 | 0.967 | First | |
| 4.0 | 1.2 × 10−4 | 0.059 | 0.990 | 0.131 | 0.962 | First | |
| SMS-GME | 0.2 | 1.0 × 10−4 | 0.076 | 0.960 | 0.031 | 0.998 | Second |
| SMSG-2 | 0.2 | 1.0 × 10−4 | 0.002 | 0.979 | 0.003 | 0.990 | Second |
 | ||
| Fig. 7 Plot of rate constant vs. initial concentration of 4-NP for SMSG-1 (catalyst dose = 4.0 g L−1, concentration of NaBH4 = 0.1 M). | ||
Kuroda et al.34 have used PMMA as a support for gold nanoparticles and the reported nitrophenol reduction rate constant was 7.2 to 7.9 × 10−3 s−1. Gao et al.37 and Fenger et al.38 have used almost same the quantity of gold but the rate is slightly more in the case of Gao et al. In another report by Koga and Kitaoka17 Au–ZnO is applied to 4-NP reduction while the dose of catalyst is 0.985 g L−1. But still its rate of the reaction is comparable to that reported in the Au/CTAB study.38 In our work, in the case of SMSG-1, the rate can be compared with the Au/graphene work.39 Some selected reports are considered for comparing the pseudo-first order rate constants and the operational conditions for 4-NP reduction (Table 2).
| Sl. no. | Catalyst | [4-NP] (M) | Dose of catalyst in terms of gold (g L−1) | [NaBH4] (M) | Rate constant | Reference |
|---|---|---|---|---|---|---|
| a Resorcinol–melamine–formaldehyde resin nanospheres. | ||||||
| 1 | Au–PMMA | 4.3 × 10−4 | 5.77 × 10−3 | 0.63 | 7.2–7.9 × 10−3 s−1 | 34 |
| 2 | Au–poly(AG-co-VP) | 6.7 × 10−4 | 2.56 × 10−4 | 0.067 | 7.36 × 10−3 s−1 | 37 |
| 3 | Au–ZnO powder | 0.5 × 10−3 | 3.28 × 10−2 | 0.05 | 7.6 × 10−3 s−1 | 17 |
| 4 | Au/graphene | 1.0 × 10−4 | 0.753 × 10−3 | 0.1 | 3.17 × 10−3 s−1 | 39 |
| 5 | Au@RMFNSa | 2 × 10−4 | 2.19 × 10−3 | 0.0056 | 33 × 10−3 s−1 | 40 |
| 6 | SMSG-1 | 0.4 × 10−4 | 5.0 × 10−2 | 0.1 | 4.22 × 10−3 s−1 | This work |
To examine the recyclability of the SMS-GME, the Au(III)-SMS was added (at a dose of 0.5 g L−1) to a mixture of 4-NP (1.0 × 10−4 M) and NaBH4 (0.1 M). The color of the material changed immediately from yellow to violet. Au(III) got reduced to Au(0) by NaBH4 in situ, and simultaneously 4-NP was reduced to 4-AP in the presence of the embryonic Au(0). In this case, the particle size is very small as it is in the formation stage. These small embryonic particles are called the growing micro-electrode (GME), and they have a very high catalytic activity. In the first cycle with the SMS-GME the reduction is found to be ∼57% with a 10 min reaction time and the rate constant is found as 0.153 mg−1 L min−1. In the second cycle the percentage reduction is decreased to ∼41% (with a 60 min reaction time duration) with a rate constant of 0.017 mg−1 L min−1. Thus the rate is ∼9 times lower when compared to that observed in the first cycle (Fig. 9b). In the second cycle the SMS-GME comprises already formed gold nanoparticles, and the material is no longer considered as a GME but it is a FGME.
To examine whether CTAB is leached from the SMSG surface, the CTAB concentration in the reaction mixture was determined after the borohydride reduction of 4-NP and no leaching of CTAB was noticed to take place. Also no Au(0) surface plasmon was observed after the reaction indicating that Au(0) was not leached during the 4-NP reduction. This suggests that the catalyst is stable. To understand whether there is any change in the crystalline nature of Au(0) after 4-NP reduction, XRD analysis of the used catalyst after the first cycle was performed. The results shown in Fig. S4† reveal that the gold catalyst still retains its crystalline character.
It is encouraging to observe that for the SMS-GME the TON and TOF values are 1.2 × 1023 molecules per g and 20.07 × 1019 molecules per g per s, which are much higher when compared to those of SMSG-1 or SMSG-2. In this study the initial 4-NP concentration applied is 1.0 × 10−4 M and the catalyst dose is 0.5 g L−1. The higher efficiency of the GME is due to the smaller particle size (which relates to a larger surface area) and the freshly evolved surface of the gold catalyst.
4. Conclusions
Silica upon treatment with CTAB under specified conditions forms an adsorbed surfactant bilayer which has the unique capability of adsolubilizing organic molecules. The effect of this micellar environment for Au(0) formation and its catalytic application on 4-NP reduction has been investigated for the first time. Gold nanoparticles supported on the surfactant-modified silica were prepared by two different methods: (1) photoactivation; (2) borohydride reduction. The XRD studies of the materials confirm the formation of Au(0) on the surface. SEM images reveal the distributions of the gold nanoparticles on the SMS surface. FTIR analysis gives information about the peaks of the supporting material, i.e. silica and CTAB. Both of the nanoparticles are applied as a catalyst for 4-NP reduction in NaBH4 medium in ambient conditions. The kinetics and the mechanism are discussed. The surfactant bilayer present in the silica surface has a great synergistic effect on both gold loading and on the catalytic activity for 4-NP reduction. The catalytic activity of the GME and FGME are compared. The rate of reduction with the SMS-GME was noted to be ∼9 times faster than the FGME, while a 0.5 g L−1 dose and 1.0 × 10−4 M 4-NP are used.Acknowledgements
The authors are thankful to IIT Kharagpur for providing the instrumental facility and financial support.References
- G. Hutchings, J. Catal., 1985, 96, 292–295 CrossRef CAS.
- M. Haruta, T. Kobayashi, H. Sano and N. Yamada, Chem. Lett., 1987, 16, 405–408 CrossRef.
- M. Haruta, N. Yamada, T. Kobayashi and S. Iijima, J. Catal., 1989, 115, 301–309 CrossRef CAS.
- Y. Chen, J. Qiu, X. Wang and J. Xiu, J. Catal., 2006, 242, 227–230 CrossRef CAS PubMed.
- A. Wolf and F. Schuth, Appl. Catal., A, 2002, 226, 1–13 CrossRef CAS.
- M. M. Schubert, S. Hackenberg, A. C. Veen, M. Muhler, V. Plzak and R. J. Behm, J. Catal., 2001, 197, 113–122 CrossRef CAS.
- T. Mitsudome and K. Kaneda, Green Chem., 2013, 15, 2636–2654 RSC.
- J. Noh and R. Meijboom, Reduction of 4-nitrophenol as a model reduction for nanocatalysis, in Application of Nanotechnology in Water Research, ed. A. K. Mishra, Scrivener Publishing LLC, 2014, ch. 13, pp. 333–406 Search PubMed.
- H. Tanaka, M. Mitsuishi and T. Miyashita, Langmuir, 2003, 19, 3103–3105 CrossRef CAS.
- S. Ivanova, C. Petit and V. Pitchon, Appl. Catal., A, 2004, 267, 191–201 CrossRef CAS PubMed.
- S. Praharaj, S. Nath, S. K. Ghosh, S. Kundu and T. Pal, Langmuir, 2004, 20, 9889–9892 CrossRef CAS PubMed.
- S. Panigrahi, S. Basu, S. Praharaj, S. Pande, S. Jana, A. Pal, S. K. Ghosh and T. Pal, J. Phys. Chem. C, 2007, 111, 4596–4605 CAS.
- L. X. Xu, C. H. He, M. Q. Zhu, K. J. Wu and Y. L. Lai, Catal. Lett., 2007, 118, 248–253 CrossRef CAS.
- S. Saha, A. Pal, S. Kundu, S. Basu and T. Pal, Langmuir, 2010, 26, 2885–2893 CrossRef CAS PubMed.
- S. Saha, A. Pal, S. Pande, S. Sarkar, S. Panigrahi and T. Pal, J. Phys. Chem. C, 2009, 113, 7553–7560 CAS.
- S. Wunder, F. Polzer, Y. Lu, Y. Mei and M. Ballauff, J. Phys. Chem. C, 2010, 114, 8814–8820 CAS.
- H. Koga and T. Kitaoka, Chem. Eng. J., 2011, 168, 420–425 CrossRef CAS PubMed.
- X. Huang, X. Liao and B. Shi, Green Chem., 2011, 13, 2801–2805 RSC.
- J. Wang, A. H. Lu, M. Li, W. Zhang, Y. S. Chen, D. X. Tian and W. C. Li, ACS Nano, 2013, 7, 4902–4910 CrossRef CAS PubMed.
- S. Koner, A. Pal and A. Adak, Desalination, 2011, 276, 142–147 CrossRef CAS PubMed.
- T. Aditya, A. Pal and T. Pal, Chem. Commun., 2015, 51, 9410–9431 RSC.
- A. V. Few and R. Ottewill, J. Colloid Sci., 1956, 11, 34–38 CrossRef CAS.
- S. Koner, A. Pal and A. Adak, Desalin. Water Treat., 2010, 22, 1–8 CrossRef CAS.
- S. Musić, N. Filipović-Vinceković and L. Sekovanić, Braz. J. Chem. Eng., 2011, 28, 89–94 Search PubMed.
- K. Esumi, Adsolubilization of organic pollutant, in Encyclopedia of Surface and Colloid Science, ed. P. Somasundaran, CRC Press, 2nd edn, 2006, vol. 1 Search PubMed.
- G. Nallathambi, T. Ramachandran, V. Rajendran and R. Palanivelu, Mater. Res., 2011, 14, 552–559 CrossRef CAS.
- S. Reddy, B. E. K. Swami and H. Jayadevappa, Electrochim. Acta, 2012, 61, 78–86 CrossRef CAS PubMed.
- X. K. Ma, N. H. Lee, H. J. Oh, J. W. Kim, C. K. Rhee, K. S. Park and S. J. Kim, Colloids Surf., A, 2010, 358, 172–176 CrossRef CAS PubMed.
- C. Huo, J. Ouyang and H. Yang, Sci. Rep., 2014, 4, 3682 Search PubMed.
- O. Yayapao, T. Thongtem, A. Phuruangrat and S. Thongtem, J. Alloys Compd., 2011, 509, 2294–2299 CrossRef CAS PubMed.
- S. Gu, S. Wunder, Y. Lu, M. Ballauff, R. Fenger, K. Rademann, B. Jaquet and A. Zaccone, J. Phys. Chem. C, 2014, 118, 18618–18625 CAS.
- M. Li and G. Chen, Nanoscale, 2013, 5, 11919–11927 RSC.
- M. L. Wang, T. T. Jiang, Y. Lu, H. J. Liu and Y. Chen, J. Mater. Chem. A, 2013, 1, 5923–5933 CAS.
- K. Kuroda, T. Ishida and M. Haruta, J. Mol. Catal. A: Chem., 2009, 298, 7–11 CrossRef CAS PubMed.
- N. C. Antonels and R. Meijboom, Langmuir, 2013, 29, 13433–13442 CrossRef CAS PubMed.
- S. Gu, J. Kaiser, G. Marzun, A. Ott, Y. Lu, M. Ballauff, A. Zaccone, S. Barcikowski and P. Wagener, Catal. Lett., 2015, 145, 1105–1112 CrossRef CAS.
- Y. Gao, X. Ding, Z. Zheng, X. Cheng and Y. Peng, Chem. Commun., 2007, 3720–3722 RSC.
- R. Fenger, E. Fertitta, H. Kirmse, A. F. Thünemann and K. Rademann, Phys. Chem. Chem. Phys., 2012, 14, 9343–9349 RSC.
- J. Li, C. Y. Liu and Y. Liu, J. Mater. Chem., 2012, 22, 8426–8430 RSC.
- S. Wang, J. Zhang, P. Yuan, Q. Sun, Y. Jia, W. Yan, Z. Chen and Q. Xu, J. Mater. Sci., 2015, 50, 1323–1332 CrossRef CAS.
- J. Belloni, Curr. Opin. Colloid Interface Sci., 1996, 1, 184–196 CrossRef CAS.
- A. Henglein, J. Phys. Chem., 1993, 97, 5457–5471 CrossRef CAS.
- N. Pradhan, A. Pal and T. Pal, Colloids Surf., A, 2002, 196, 247–257 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16655a |
| This journal is © The Royal Society of Chemistry 2015 |