Large-area MoS2 thin layers directly synthesized on Pyramid-Si substrate for surface-enhanced Raman scattering
Abstract
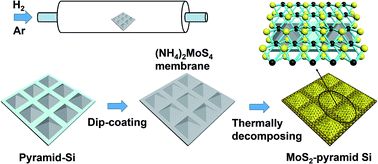
In our work, we directly synthesized few layer MoS2 on a pyramid-Si substrate to fabricate a surface-enhanced Raman scattering (SERS) substrate via thermally decomposing the precursor of ammonium thiomolybdate ((NH4)2MoS4). Scanning electron microscopy (SEM), atomic force microscopy (AFM), X-ray diffraction (XRD) and Raman spectra are employed to characterize the as-grown MoS2 layers. Adenosine and cytidine were selected as the probe molecules to investigate the SERS ability of the MoS2-pyramid-Si substrate, and have shown that the MoS2-pyramid-Si substrate can prominently suppress photobleaching and fluorescence of the probe molecule. Compared with the MoS2-flat-Si substrate (MoS2 layers synthesized on flat-Si substrate), the MoS2-pyramid-Si substrate has more significant SERS ability. The minimum detected concentration of both adenosine and cytidine on the MoS2-pyramid-Si substrate can reach 10−6 M. Importantly, the linear relationship between the Raman intensity and the concentration of adenosine or cytidine can apply to the bimolecular detection. This work may provide a new opportunity for the study of the chemistry mechanism (CM) and novel SERS substrate fabrication.
- This article is part of the themed collection: Surface enhanced Raman Spectroscopy: Editors collection for RSC Advances

 Please wait while we load your content...
Please wait while we load your content...