Improvement in the performance of a zinc ion-selective potentiometric sensor using modified core/shell Fe3O4@SiO2 nanoparticles
A. A. Bazrafshana,
S. Hajati*b and
M. Ghaedi*a
aChemistry Department, Yasouj University, Yasouj 75918-74831, Iran. E-mail: m_ghaedi@mail.yu.ac.ir; m_ghaedi@yahoo.com; Fax: +98 74 33223048; Tel: +98 74 33223048
bDepartment of Physics, Yasouj University, Yasouj 75918-74831, Iran. E-mail: hajati@mail.yu.ac.ir
First published on 27th November 2015
Abstract
A novel, simple, accurate and sensitive zinc ion-selective potentiometric sensor was fabricated by modifying the surface of Fe3O4@SiO2 nanoparticles using a ligand (L) prepared by a coupled reaction between (3-aminopropyl)trimethoxysilan (APTMS) and 2-hydroxy-3-methoxybenzaldehyde (2-H-3-MBA). The prepared Fe3O4 nanoparticles were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray powder diffraction (XRD) and Fourier transform infrared (FTIR). In addition, Fe3O4@SiO2, Fe3O4@SiO2–APTMS, and Fe3O4@SiO2–L were characterized by FTIR. X-ray photoelectron spectroscopy was used to confirm the surface modification of the Fe3O4@SiO2 nanoparticles. The effects of individual variables such as the amount of graphite powder, Fe3O4@SiO2–L and sodium tetraphenylborate (NaTPB) used in the composition of the carbon paste electrode (CPE) in addition to their possible interactions were investigated and optimized using a central composite design (CCD) under response surface methodology (RSM). The optimal values of graphite powder, Fe3O4@SiO2–L and NaTPB in a proper amount of paraffin oil obtained were 72, 15 and 6 mg, respectively. The prepared electrode applied for sensing Zn2+ ions demonstrated a linear range of 2.5 × 10−6 to 1.00 × 10−1 mol L−1. The detection limit, slope and response time of this sensor were found to be 10−6 mol L−1, 29.451 and 14 s, respectively. The potentiometric response of the prepared electrode based on Fe3O4@SiO2–L was independent of the pH of the test solution over the pH range of 4–6, which is an advantage of this electrode having a neutral working pH. This sensor was successfully used for the sensitive determination of trace amounts of Zn2+ in samples of water. The moderate selectivity coefficient evaluated by the fixed interference method (FIM) indicated an efficient discriminating ability of the proposed electrode for Zn2+ ion assessment. It was also found to be well repeatable and reproducible.
1. Introduction
The environment is being seriously contaminated by heavy metals and thus is the focus of attention all over the world.1 Zinc ions, with a quantity in serum being about 10 μM, is the most abundant heavy metal ion in the human body. Zinc ions are an important constituent of a number of enzymes such as carbonic anhydrase and matrix metalloproteinase,2 and they help in the maintenance of the structural characteristics of gene transcription proteins such as zinc finger proteins, etc.3 However, an excess of zinc ions as an environmental pollutant can be toxic and decreases the soil microbial activity.4Zinc is widely used in various industries such as electroplating, paint, food and agricultural waste, chemical and pharmaceutical and thus it is present in the waste and effluent of these plants. Its uptake by humans above a certain level causes pulmonary diseases, fever, nausea, anaemia, and renal failure. Therefore, the determination of zinc ions is of high importance. Many analytical techniques including UV-vis spectroscopy,5,6 flame atomic absorption spectrometry,7 inductively coupled plasma atomic emission spectrometry (ICPAES)8 and fluorescence methods9–13 have been used for the determination of zinc ions.
Electrochemical methods that are highly sensitive and selective in their responses have been widely used to determine various substances.14–17 Among these methods, potentiometry is an easy and inexpensive technique that is applied in many clinical, environmental and toxicological analyses. Two-electrode potentiometric sensors as an important class of electrochemical sensor perform based on the observed potential response against the activity of the analyte species. In simple and efficient potentiometric methods, ion sensors that are called ion-selective electrodes (ISEs) are used as indicator electrodes for the determination of heavy metal ions and anions.18–25 Among these electrodes, ISEs based on carbon paste, which are easy to structure, have simple instrumentation, a fast response, a wide dynamic range, reasonable selectivity, an easily renewable surface, are low cost and compatible with various types of modifiers,26 have been widely used as suitable matrices for the construction of the sensors. Selection of suitable modifiers can improve the electrode surface state, which may cause a significant enhancement in the target signals.26
Nanomaterials, including nanotubes, nanowires, nanofibers, nanorods, nanoparticles, nanocomposites27,28 and other nanostructured materials have been widely applied to construct a variety of chemosensors and biosensors with exceptional physical and chemical properties such as a large surface area to volume ratio, good conductivity, excellent electrocatalytic activity and high mechanical strength.29 Recently, nanomaterials have been gradually introduced into potentiometric sensors. These nanomaterials are suitable for the construction of sensitive electrochemical sensors. Significant advances have been achieved in synthetic methodologies. The enhancement in selectivity and sensitivity of sensors for accurate sensing may be achieved by decorating the nanomaterials with polymers and ligands (e.g., Schiff bases).
In addition, carbon paste made by a mixture of carbon (graphite) powder and a binder (pasting liquid) is suitable for the fabrication of many electrometric sensors for analytical purposes.30,31 The operation of such electrodes depends on the properties of the modifiers used to enhance the selectivity of the sensor towards the target species.
In this work, a novel, simple, accurate and sensitive zinc ion-selective potentiometric sensor was fabricated by modifying the surface of Fe3O4@SiO2 nanoparticles using a ligand (L) prepared by sequential reaction between (3-aminopropyl)trimethoxysilan (APTMS) and 2-hydroxy-3-methoxybenzaldehyde (2-H-3-MBA). Response surface methodology (RSM) was applied to optimize the responses versus variables such as the amount of graphite powder, Fe3O4@SiO2–L and sodium tetraphenylborate (NaTPB) used in the composition of the CPE by performing the least number of experimental runs. In addition, the effects of interaction between the variables on the responses were investigated by RSM. The sensor characteristics such as the linear range, response time, detection limit, selectivity, sensitivity, repeatability and reproducibility were investigated.32
2. Experimental
2.1. Chemicals and instruments
Iron(III) chloride (FeCl3), iron(II) chloride tetrahydrate (FeCl2·4H2O), NH4OH (25%, v/v) tetraethyl orthosilicate (TEOS), trimethoxy silyl propyl amine (APTMS), 2-hydroxy-3-methoxybenzaldehyde (2-H-3-MBA), methanol, graphite powder, high purity paraffin oil, sodium tetraphenylborate, metallic salts and tetrahydrofuran (THF) were purchased from Merck (Darmstadt, Germany) and used without further treatment. All the metal nitrate solutions were freshly prepared by accurate dilution of their stock solution (0.1 M) using double distilled water.All potential measurements were carried out by a pH/mV meter (Zag Chimi, Iran) using the fabricated sensor together with a double junction Ag/AgCl reference electrode. The pH measurements were carried out using a pH/ion meter model-686 (Metrohm, Swiss). X-ray powder diffraction (XRD) spectra were taken using an X’pert diffractometer of Philips Company (Netherlands) with monochromatized Cu Kα radiation. The morphology of the nanomaterials was characterized using scanning electron microscopy (SEM, KYKY-EM3200, China) and a transmission electron micrograph was obtained using a Philips CM200 FEG\ST Lorentz electron microscope with a field emission gun at an acceleration voltage of 200 kV. FTIR spectra were recorded using a JASCO-FTIR680 (Japan) instrument over 4000–400 cm−1. X-ray photoelectron spectroscopy (XPS) data were obtained using an 8025-BesTec twin anode XR3E2 X-ray source system (Germany). XPS data were taken using an Al Kα (1486.6 eV) X-ray source operated at 15 kV.
2.2. Synthesis of Fe3O4 nanoparticles
For the preparation of Fe3O4 nanoparticles, FeCl3 (11.2 mmol) and FeCl2·4H2O (5.6 mmol) were separately dissolved in 50 mL double distilled water followed by mixing them. The temperature of the solution was adjusted to 80 °C. Then, while stirring under nitrogen atmosphere, 15 mL of NH4OH (25%, v/v) was added dropwise over 30 min. After 120 min for the reaction had elapsed, the color of the bulk solution changed from orange to black. The magnetite precipitates were washed several times with deionized water and ethanol. The black Fe3O4 nanoparticles were separated using a permanent magnet. The synthesized Fe3O4 nanoparticles were dried at 70 °C for 12 h.2.3. Preparation of nanostructured silica-coated magnetite (Fe3O4@SiO2)
Fe3O4 nanoparticles were covered with a silica coating using TEOS. For this purpose, 300 mg of Fe3O4 nanoparticles was placed in a beaker containing 50 mL of methanol and 8 mL of deionized water followed by ultrasonication for 30 min. Then it was transferred to a double-neck flask and 5 mL of NH4OH (25%, v/v) and 2 mL of TEOS were added to it. The suspension was stirred for 36 h under nitrogen atmosphere at room temperature. Finally, the silica-decorated magnetic nanoparticles (Fe3O4@SiO2) were washed using diluted hydrochloric acid and water and were separated by a permanent magnet and dried at 70 °C for 8 h.2.4. Surface modification of Fe3O4@SiO2 using APTMS
400 mg of Fe3O4@SiO2 nanoparticles was chemically modified by 3.5 mL of APTMS in 40 mL methanol solution by refluxing for 40 h at 65 °C. The Fe3O4@SiO2–APTMS was collected by an external magnetic field and then dried at 70 °C for 10 h.2.5. Preparation of Fe3O4@SiO2–L
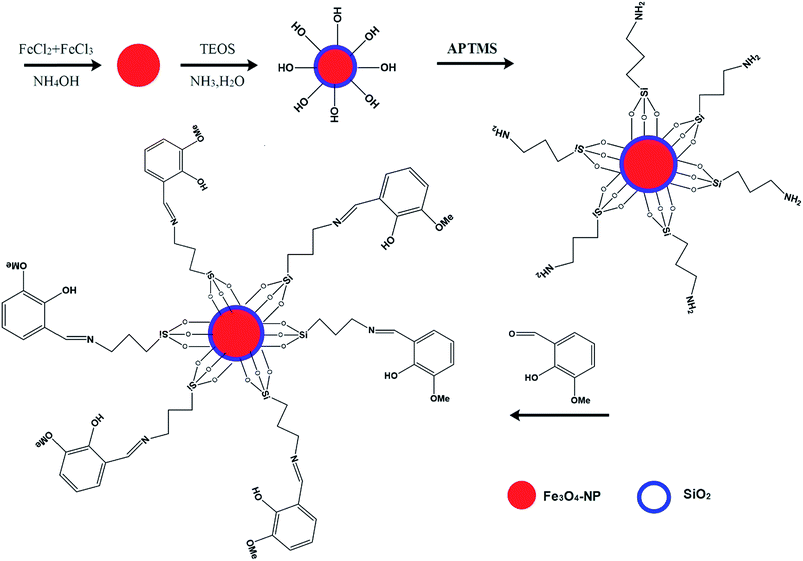
The Schiff base (L) chelate generation on the surface of Fe3O4@SiO2 was carried out by the addition of 0.300 g of 2-hydroxy-3-methoxybenzaldehyde (2-H-3-MBA) to 40 mL methanol containing 0.6250 g Fe3O4@SiO2–APTMS obtained from the previous stage. Then the reaction mixture was refluxed for 36 h at 65 °C. Finally, Fe3O4@SiO2–L was collected by an external magnetic field and dried at 70 °C for 4 h. The schematic diagram of Fe3O4@SiO2–L preparation is shown in Fig. 1.2.6. Electrode preparation
A Teflon holder with a hole of diameter 5.0 mm and depth of 3 mm at one end was fabricated for filling the carbon paste as the electrode body. Carbon paste was prepared by mixing the graphite powder with the desired weights of Fe3O4@SiO2–L and NaTPB to get different compositions. The mixture was homogenized in THF at room temperature and the THF was allowed to be completely evaporated. Then paraffin oil was homogeneously mixed by agate mortar and pestle sets to get different compositions. The prepared paste was then packed into the hole of the Teflon holder. Electrical contact to the paste was established via inserting a thin copper wire. A fresh electrode surface was obtained by squeezing it out. Additionally it was polished on paper until a shiny surface was obtained. The prepared pastes were used directly for potentiometric measurements without any preconditioning.2.7. EMF measurements
All the EMF measurements were carried out relative to an Ag/AgCl electrode by a digital pH/mV meter with the cell assembly of Ag/AgCl(s), KCl (3 mol L−1) | sample solution | developed carbon paste electrode.A calibration graph was prepared by plotting the potential of the cell E (mV) versus log[Zn2+]. All measurements were carried out at 25 °C. The performance of the electrodes was investigated by measuring the EMFs of the Zn2+ ion solutions with a desired concentration in the range of 1 × 10−9 to 1 × 10−1 M and by serial dilution. In each solution, while stirring after reaching the equilibrium, the potential was recorded and plotted.
3. Results and discussion
3.1. General consideration
The Nernst equation provides the fundamental principle of all potentiometric transducers. According to this equation, the potential changes are logarithmically proportional to the specific ion activity. It is assumed that the interfacial ion transfer and the complexation processes are relatively fast and consequently the equilibrium is held at the interface.333.2. Characterization of magnetite and modified magnetite nanoparticles
The Fe3O4 nanoparticles were characterized by SEM, TEM and powder XRD. The SEM image of the Fe3O4 nanoparticles (Fig. 2a) shows a size distribution of 20–50 nm, which is confirmed by the TEM micrograph (Fig. 2b). The TEM image of the modified Fe3O4@SiO2 is shown in Fig. 2c. The typical XRD pattern taken from the magnetite nanoparticles is shown in Fig. 2d, which is in accordance with the standard characteristics of Fe3O4 (powder diffraction file, JCPDS card no. 00-003-0863).FTIR spectra of Fe3O4, Fe3O4@SiO2, Fe3O4@SiO2–APTMS and Fe3O4@SiO2–L were recorded and used to investigate the surface modification of the Fe3O4 nanoparticles (Fig. 2e). All the spectra obviously show one strong absorption peak at the low frequency region corresponding to the iron oxide skeleton (the Fe–O stretching band appears at 590 cm−1) and one broad absorption band which is attributed to the stretching vibrations of O–H, appearing at about 3419 cm−1. The silica-coated magnetite shows a band at 1098 cm−1 corresponding to the stretching vibrations of Si–O–Si. The FTIR spectrum of Fe3O4@SiO2–L presents an absorption peak at 1631.4 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, confirming the formation of the L (Schiff base) on the surface of Fe3O4@SiO2.
N bond, confirming the formation of the L (Schiff base) on the surface of Fe3O4@SiO2.
Fig. 2f shows the XPS survey spectrum of Fe3O4@SiO2–L. As mentioned above, the FTIR peak at 590 cm−1 originates from the Fe–O bond, which is an indication of the formation of Fe3O4. However, no XPS signal from Fe was observed which implies that the modified SiO2 shell is thicker than the sampling depth of XPS. This thick modified SiO2 shell is confirmed by the TEM image of the modified Fe3O4@SiO2 (Fig. 2c). C 1s and N 1s peaks originating from the ligand located on the surface of Fe3O4@SiO2 are observed (Fig. 2f). It is worth noting that the charging effect was compensated by using the C 1s binding energy. Savitzky–Golay filtering was performed to smooth the spectra.34,35
3.3. Preliminary investigations on the CPE
High selectivity and improved CPE figures of merit need strong interaction between the carrier and target ions. To estimate the selective interaction of the proposed CPE with the ions, a series of similar electrodes based on 67 mg of graphite powder, 10 mg of carrier, 3 mg of cationic additive (NaTPB) and a suitable amount of paraffin were prepared. Then an investigation on their responses toward various metal ions in the concentration range of 1.0 × 10−2 to 1.0 × 10−7 M was performed. The obtained results confirmed the most sensitive response for Zn2+ ions over a wide concentration range compared to the other ions studied.3.4. Central composite design (CCD) for the carbon paste electrode composition
In CCD under response surface methodology (RSM), random experiments were designed using the software STATISTICA (Table 1) and performed to minimize the effect of uncontrolled variables. The three variables including the amount of graphite powder, Fe3O4@SiO2–L and NaTPB were set at three levels of low, central and high (Table 1). RSM was used to investigate the influence of these variables and their possible interactions on the Nernstian response of the electrode to Zn2+ and thus to optimize and improve the composition of the carbon paste electrode. The amount of graphite powder, Fe3O4@SiO2–L and NaTPB was denoted by A, B and C, respectively.| Factors | Units | −1 level | +1 level | −Alpha | +Alpha |
|---|---|---|---|---|---|
| Graphite powder (A) | mg | 55 | 85 | 40 | 100 |
| Fe3O4@SiO2–L (B) | mg | 5 | 15 | 0 | 20 |
| NaTPB (C) | mg | 2 | 6 | 0 | 8 |
| Std | Run | Block | A | B | C | Slope |
|---|---|---|---|---|---|---|
| 4 | 1 | Block 1 | 70.00 | 10.00 | 4.00 | 22.42 |
| 11 | 2 | Block 1 | 55.00 | 5.00 | 2.00 | 18.809 |
| 2 | 3 | Block 1 | 70.00 | 10.00 | 4.00 | 22.54 |
| 3 | 4 | Block 1 | 85.00 | 15.00 | 2.00 | 20.7 |
| 7 | 5 | Block 1 | 55.00 | 15.00 | 6.00 | 26.894 |
| 5 | 6 | Block 1 | 40.00 | 10.00 | 4.00 | 15.098 |
| 1 | 7 | Block 1 | 70.00 | 10.00 | 0.00 | 15.855 |
| 15 | 8 | Block 1 | 70.00 | 10.00 | 4.00 | 21.5 |
| 14 | 9 | Block 1 | 70.00 | 10.00 | 8.00 | 20.61 |
| 8 | 10 | Block 1 | 70.00 | 0.00 | 4.00 | 11.8111 |
| 6 | 11 | Block 1 | 70.00 | 20.00 | 4.00 | 29.629 |
| 13 | 12 | Block 1 | 85.00 | 5.00 | 6.00 | 9.3604 |
| 9 | 13 | Block 1 | 100.00 | 10.00 | 4.00 | 11.6686 |
| 12 | 14 | Block 1 | 70.00 | 10.00 | 4.00 | 22.54 |
| 10 | 15 | Block 1 | 70.00 | 10.00 | 4.00 | 22.54 |
Table 2 shows the results of the analysis of variance (ANOVA) and the regression coefficients, which suggest that the significance of the applied quadratic model has a p-value of less than 0.05.
| Slope = −11.599 + 1.108A − 1.893B + 0.487C + 0.027AB − 0.013AC + 0.305BC − 0.010A2 − 0.015B2 − 0.251C2 | (1) |
| Source | Sum of squares | Dfa | Mean square | F value | p-value prob > F | |
|---|---|---|---|---|---|---|
| a Df: degrees of freedom. | ||||||
| Model | 455.53 | 9 | 50.61 | 241.76 | <0.0001 | Significant |
| A | 5.88 | 1 | 5.88 | 28.09 | 0.0032 | |
| B | 158.74 | 1 | 158.74 | 758.23 | <0.0001 | |
| C | 11.31 | 1 | 11.31 | 54.00 | 0.0007 | |
| AB | 10.69 | 1 | 10.69 | 51.07 | 0.0008 | |
| AC | 0.43 | 1 | 0.43 | 2.06 | 0.2111 | |
| BC | 24.86 | 1 | 24.86 | 118.75 | 0.0001 | |
| A2 | 114.88 | 1 | 114.88 | 548.73 | <0.0001 | |
| B2 | 3.42 | 1 | 3.42 | 16.32 | 0.0099 | |
| C2 | 23.58 | 1 | 23.58 | 112.63 | 0.0001 | |
| Residual | 1.05 | 5 | 0.21 | |||
| Lack of fit | 0.22 | 1 | 0.22 | 1.06 | 0.3606 | Not significant |
| Pure error | 0.83 | 4 | 0.21 | |||
| Cor total | 456.58 | 14 | ||||
The lack of fit (LOF) indicates the variation of the data nearby the fitted model, which is a criterion for the suitability of the model. As seen in Table 2, the p-value of the LOF is 0.3606, which is larger than 0.05 and implies that the LOF is not significant and the model fits the response well. R2 is a measure of the amount of deviation around the mean explained by the model. The large adjusted R2 values (0.9936) indicate a good relationship between the experimental data and the fitted model and thus the suitability of the model. The plot of the predicted values of response (slope) versus the observed values (Table 1) is shown in Fig. 3a which demonstrates a good fit. This shows a normal distribution of error around the mean, which expresses the good applicability of the model for the explanation of the experimental data.
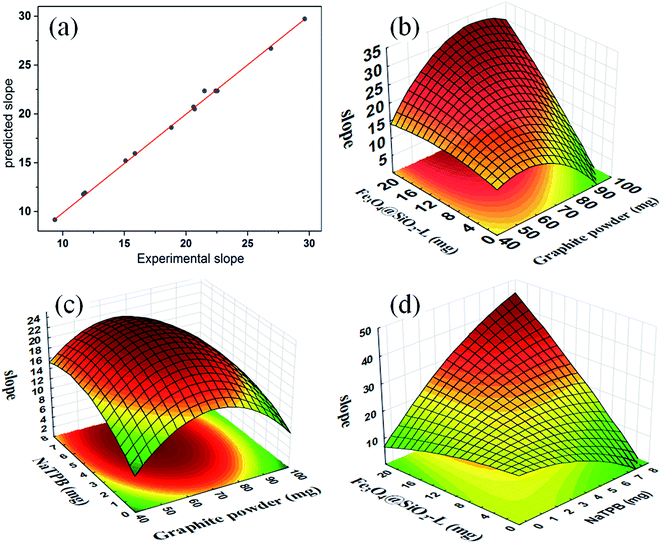 | ||
| Fig. 3 The predicted versus experimental data of the normalized slope (a), and response surfaces: the slope versus variables (b)–(d). | ||
3.5. Response surface methodology and optimization
Response surface methodology (RSM) was used to optimize the critical factors and describe the nature of the response surface in the experiments. The typical 3D response surface plots of the slope as the response versus the significant variables were obtained and are shown in Fig. 3b–d, the curvatures of which indicate the interaction between the variables involved. Fig. 3b shows that at low amounts of graphite powder, no significant increase in the slope is obtained by increasing the amount of Fe3O4@SiO2–L. It also shows that at low amounts of Fe3O4@SiO2–L, the slope decreases by increasing the amount of graphite powder. Moreover, a concurrent increase in the amount of graphite powder and Fe3O4@SiO2–L increases the slope. Fig. 3c shows the interaction between NaTPB and graphite powder. Fig. 3d shows that at low amounts of NaTPB, a mild variation in the slope is observed while the amount of Fe3O4@SiO2–L increases. At low amounts of Fe3O4@SiO2–L, the slope slowly decreases while the amount of NaTPB increases. Furthermore, the rate of increase in the slope gets higher while a simultaneous increase in the amount of NaTPB and Fe3O4@SiO2–L occurs.An accurate consideration of eqn (1) and the desirability function (DF) makes it possible to obtain the best slope by running the least number of experiments with high repeatability. The presence of Fe3O4@SiO2–L resulted in a development in the kinetics of the electrode process and sensitivity of the potentiometric measurements. The profiles for the predicted values and desirability were used for the optimization process. The scale must be maximized in the range of 0.0 (undesirable) to 1.0 (very desirable) following the best and an efficient selection of variables. For a sufficient amount of paraffin, the optimum values of the amount of graphite powder, Fe3O4@SiO2–L and NaTPB were found to be 72, 15 and 6 mg, respectively, at which the slope obtained was 29.717 with a high desirability of 1.00. To validate this prediction, similar experiments at optimum conditions were performed which consistently resulted in 29.415 mV per decade of concentration for the slope with five orders of magnitude for the linear range.
3.6. Effect of pH on the electrode response
The effect of the pH of the test solution on the electrode response was studied in Zn(NO3)2 solutions (1.0 × 10−3 M and 1.0 × 10−5 M) and the results are presented in Fig. 4. pH adjustments in solutions were performed using nitric acid or sodium hydroxide solutions. It was seen that the electrode response is hardly affected by pH in the range of 4.0–6.0. The potential changes observed at lower pH values could be due to the protonation of the ion carrier and/or various functional groups of the graphite powder. At higher pH values, because of the formation of some hydroxy complexes or hydroxide precipitate of Zn2+ ions in solution and/or because of a probable change in the stoichiometry of the metal chelate species, a drift in response was achieved. Subsequently, the pH of the Zn2+ ion solution was set to be about 5.5 as the working pH without the addition of a buffer.3.7. Dynamic range
As seen in Fig. 5a, the calibration curve is linear over the concentration range of 2.5 × 10−6 to 1.0 × 10−1 mol L−1. In this work, the detection limit of the modified CPE obtained was 1.0 × 10−6 mol L−1 which was calculated by extrapolating the two segments of the calibration curve in Fig. 5a.3.8. Response time
The response time of an ion-selective electrode is also an important factor for any analytical application. Response time is the average time required for the electrode to reach a potential response of the final equilibrium value ±1 mV (ref. 36) or to achieve 90% of the final equilibrium value.37 The response time obtained for Zn2+ in the concentration range of 1.0 × 10−6 to 1.0 × 10−2 mol L−1 is presented in Fig. 5b. It was found to be 14 s after successive immersions in a series of Zn2+ solutions, each having a 10-fold concentration difference.3.9. Repeatability and reproducibility
The repeatability of Zn2+ ion measurement using the prepared electrode was investigated by washing the electrode only with distilled water and more than 15 successive potentiometric measurements were performed on a 1.0 × 10−3 M Zn2+ solution and vice versa. The washing step required for the electrode to return to the starting potential was carried out during ∼1 min with a standard deviation of ±1.83 mV in the potential. Three similar electrodes were reproduced at optimum conditions. Their linear ranges were found to be 1.0 × 10−6 to 1.0 × 10−1 M and their corresponding slope values were 29.464, 29.465 and 29.318 (mV per decade concentration) with R2 values of 0.9974, 0.9865 and 0.9954, respectively (Fig. 6), which strongly supports its good reproducibility.3.10. Potentiometric selectivity and lifetime
The potentiometric selectivity coefficient of an electrode is defined by its relative response for the primary ion over other ions present in the solution. The IUPAC recommended the use of two different procedures of SSM and FIM to determine the Nicolskii coefficients of ion-selective electrodes.38 In the FIM, an entire calibration curve is measured for the analyte ion in a constant interfering ion background, aj(BG). The linear response curve of the electrode as a function of the analyte ion activity is extrapolated until, at the lower detection limit ai(DL), it intersects with the observed potential for the background alone. The FIM coefficient (KFIMIJ) is calculated from the obtained ratio of the lower detection limit to the interfering ion activity which is implemented in the following equation:
KFIMIJ = ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ai(DL)/aj(BG)Zi/Zj ai(DL)/aj(BG)Zi/Zj
| (2) |
| Interfering (j) | −log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KFIMIJ J = 0.001 (M) KFIMIJ J = 0.001 (M) |
|---|---|
| Na+ | 2.5 |
| K+ | 2.2 |
| Ca2+ | 2.5 |
| Pb2+ | 0.3 |
| Cu2+ | −2 |
| Cd2+ | 1.2 |
| Co2+ | 2.4 |
| Ni2+ | 2.7 |
| Mn2+ | 2.3 |
| Al3+ | 1.1 |
3.11. Analytical applications
To evaluate the applicability of the Zn2+ sensor for real samples, an attempt was made to determine Zn2+ ions in several prepared water samples. These samples were prepared by adding Zn2+ ions with a certain concentration in distilled water. Also, to study the applicability of the electrode for environmental samples, the proposed electrode was applied to the recovery of Zn2+ ions from a tap water sample. These results are summarized in Table 4, and indicate good recoveries.Table 5 shows a comparison of the proposed zinc ion sensor based on the Fe3O4@SiO2–L nanostructure with reported zinc ion sensors. The performance of the present zinc ion sensor is better than that of the reported zinc ion sensors40,41 because of the three-dimensional network of the Fe3O4@SiO2 nanostructure grafted by the Schiff base.
4. Conclusions
This work reported a new nanocomposite-based carbon paste electrode using a feasible method to construct a selective potentiometric sensor for the determination of Zn2+. Nanostructured Fe3O4@SiO2 was successfully prepared and grafted by a Schiff base. The influence of the amount of graphite powder, Fe3O4@SiO2–L and sodium tetraphenylborate (NaTPB) used in the composition of the carbon paste electrode was investigated by response surface methodology and their optimal values were found to be 72, 15 and 6 mg, respectively. Then the optimized electrode was used as a novel chemically modified carbon paste electrode for selective zinc ion determination over the concentration range of 2.5 × 10−6 to 1.0 × 10−1 M with a short response time (14 s). The electrode exhibited the best performance in terms of the working concentration range, Nernstian slope (29.451), working pH (neutral), and response time with a good detection limit, high repeatability and reproducibility.References
- J. O. Nriagu and J. M. Pacyna, Nature, 1988, 333, 134–139 CrossRef CAS PubMed.
- M. A. Abbasi, Z. H. Ibupoto, M. Hussain, Y. Khan, A. Khan, O. Nur and M. Willander, Sensors, 2012, 12, 15424–15437 CrossRef CAS PubMed.
- M. M. Parker, F. L. Humaller and D. J. Mahler, Clin. Chem., 1967, 13, 40–48 CAS.
- J. Mertens, F. Degryse, D. Springael and E. Smolders, Environ. Sci. Technol., 2007, 41, 2992–2997 CrossRef CAS PubMed.
- P. Kaur, S. Kaur, A. Mahajan and K. Singh, Inorg. Chem. Commun., 2008, 11, 626–629 CrossRef CAS.
- Y. Xu, Y. Zhou, W. Ma, S. Wang and S. Li, Appl. Surf. Sci., 2013, 276, 705–710 CrossRef CAS.
- Q. Li, X. Zhao, Q. Lv and G. Liu, Sep. Purif. Technol., 2007, 55, 76–81 CrossRef CAS.
- P. Wilhartitz, S. Dreer, R. Krismer and O. Bobleter, Microchim. Acta, 1997, 125, 45–52 CrossRef CAS.
- X. Zeng, Y. Zhou, W. Ma, S. Wang, K. Xie, J. Wu and K. Wei, Chem. Eng. J., 2014, 244, 75–81 CrossRef CAS.
- Y. Wang, X. Peng, J. Shi, X. Tang, J. Jiang and W. Liu, Nanoscale Res. Lett., 2012, 7, 1–13 CrossRef PubMed.
- X. Tian, Z. Dong, R. Wang and J. Ma, Sens. Actuators, B, 2013, 183, 446–453 CrossRef CAS.
- M. Hosseini, Z. Vaezi, M. R. Ganjali, F. Faridbod, S. D. Abkenar, K. Alizadeh and M. Salavati-Niasari, Spectrochim. Acta, Part A, 2010, 75, 978–982 CrossRef PubMed.
- Q. J. Ma, X. B. Zhang, Y. Zhao, C. Y. Li, Z. X. Han, G. L. Shen and R. Q. Yu, Spectrochim. Acta, Part A, 2009, 71, 1683–1687 CrossRef PubMed.
- V. K. Gupta, R. Jain, K. Radhapyari, N. Jadon and S. Agarwal, Anal. Biochem., 2011, 408, 179–196 CrossRef CAS.
- H. Khani, M. K. Rofouei, P. Arab, V. K. Gupta and Z. Vafaei, J. Hazard. Mater., 2010, 183, 402–410 CrossRef CAS PubMed.
- A. Afkhami, R. Moosavi and T. Madrakian, J. Electrochem. Soc., 2013, 160, 775–781 CrossRef.
- H. Bagheri, A. Afkhami, Y. Panahi, H. Khoshsafar and A. Shirzadmehr, Mater. Sci. Eng., 2014, 37, 264–270 CrossRef CAS.
- V. K. Gupta, M. R. Ganjali, P. Norouzi, H. Khani, A. Nayak and S. Agarwal, Crit. Rev. Anal. Chem., 2011, 41, 282–313 CrossRef CAS.
- V. K. Gupta, L. P. Singh, R. Singh, N. Upadhyay, S. P. Kaur and B. Sethi, J. Mol. Liq., 2012, 174, 11–16 CrossRef CAS.
- V. K. Gupta, B. Sethi, R. A. Sharma, S. Agarwal and A. Bharti, J. Mol. Liq., 2013, 177, 114–118 CrossRef CAS.
- M. Ghaedi, S. Y. Shajaripour-Jaberi, S. Hajati, M. Montazerozohori, A. Asfaram, B. Mirtamizdoust and M. Zare, IEEE Sens. J., 2015, 15, 2882–2890 CrossRef CAS.
- M. Ghaedi, S. Y. Shajaripour-Jaberi, S. Hajati, M. Montazerozohori, A. Asfaram and M. Zare, IEEE Sens. J., 2015, 15, 2974–2983 CrossRef CAS.
- M. Ghaedi, A. Shokrollahi, A. Salimibeni, S. Joybar, S. Noshadi, F. Mofazzal and S. Khodadoust, IEEE Sens. J., 2011, 11, 2129–2136 CrossRef CAS.
- M. Ghaedi, M. Montazerozohori, Z. Andikaey, A. Shokrollahi, S. Khodadoust, M. Behfar and S. Sharifi, Int. J. Electrochem. Sci., 2011, 6, 4127–4140 CAS.
- M. Ghaedi, M. Montazerozohori, K. Mortazavi, M. Behfar and F. Marahel, Int. J. Electrochem. Sci., 2011, 6, 682–6698 Search PubMed.
- H. Bagheri, A. Afkhami, A. Shirzadmehr and H. Khoshsafar, J. Mol. Liq., 2014, 197, 52–57 CrossRef CAS.
- H. L. Ding, Y. X. Zhang, S. Wang, J. M. Xu, S. C. Xu and G. H. Li, Chem. Mater., 2012, 24, 4572–4580 CrossRef CAS.
- Y. Zhang, S. Pan, X. Teng, Y. Luo and G. Li, J. Phys. Chem., 2008, 112, 9623–9626 CrossRef CAS.
- T. Asefa, C. T. Duncan and K. K. Sharma, Analyst, 2009, 134, 1980–1990 RSC.
- K. Kalcher, J. M. Kaufmann, J. Wang, I. Švancara, K. Vytřas, C. Neuhold and Z. Yang, Electroanal., 1995, 7, 5–22 CrossRef CAS.
- J. S. Yeom, M. S. Won and Y. B. Shim, J. Electroanal. Chem., 1999, 463, 16–23 CrossRef.
- M. Mazloum-Ardakani, H. Beitollah, M. K. Amini, F. Mirkhalaf and M. Abdollahi- Alibeik, Sens. Actuators, B, 2010, 151, 243–249 CrossRef CAS.
- M. Javanbakht, A. Badiei, M. R. Ganjali, P. Norouzi, A. Hasheminasab and M. abdouss, Anal. Chim. Acta, 2007, 601, 172–182 CrossRef CAS PubMed.
- S. Hajati, S. Tougaard, J. Walton and N. Fairley, Surf. Sci., 2008, 602, 3064–3070 CrossRef CAS.
- A. Savitzky and M. J. E. Golay, Anal. Chem., 1964, 36, 1627–1639 CrossRef CAS.
- M. Javanbakht, S. Eynollahi Fard, A. Mohammadi, M. Abdouss, M. R. Ganjali, P. Norouzi and L. Safaraliee, Anal. Chim. Acta, 2008, 612, 65–74 CrossRef CAS.
- V. K. Gupta, S. Chandra and R. Mangla, Sens. Actuators, B, 2002, 86, 235–241 CrossRef CAS.
- G. G. Guilbault, R. A. Durst, M. S. Frant, H. Freiser, E. H. Hansen, T. S. Light, E. Pungor, G. Rechnitz, N. M. Rice, T. J. Rohm, W. Simon and J. D. R. Thomas, Pure Appl. Chem., 1976, 48, 127–132 Search PubMed.
- M. Ghaedi, M. Montazerozohori, M. Behfar, S. Khodadoust, Z. Andikaey and M. Nejati Biareh, Sens. Lett., 2011, 9, 1718–1725 CrossRef CAS.
- V. K. Gupta, A. K. Jain, R. Mangla and P. Kumar, Electroanal., 2001, 13, 1036–1040 CrossRef CAS.
- Z. H. Ibupoto, S. M. U. Ali, C. O. Chey, K. Khun, O. Nur and M. Willander, J. Appl. Phys., 2011, 110, 104702–104707 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |