Synthesis of g-C3N4 nanosheet/Au@Ag nanoparticle hybrids as SERS probes for cancer cell diagnostics†
Jianping Wangab,
Renyong Liua,
Cheng Zhangab,
Guangmei Hana,
Jun Zhaoa,
Bianhua Liu*a,
Changlong Jiang*a and
Zhongping Zhangac
aInstitute of Intelligent Machines, Chinese Academy of Sciences, Hefei, Anhui 230031, China. E-mail: cljiang@iim.ac.cn; zpzhang@iim.ac.cn
bDepartment of Chemistry, University of Science & Technology of China, Hefei, Anhui 230026, China
cState Key Laboratory of Transducer Technology Chinese Academy of Sciences, Hefei, Anhui 230031, China
First published on 30th September 2015
Abstract
Chemical sensing for the convenient detection of cancer cells has been widely explored with the use of various sensing materials and techniques, but it is still a challenge to achieve ultrasensitive, simple, rapid and inexpensive detection of cancer cells. Herein, we report a surface-enhanced Raman scattering (SERS) method for the detection of cancer cells in situ. In our work, ultrathin g-C3N4 nanosheet/Au@AgNP hybrids (g-C3N4/Au@AgNPs) were fabricated using a self-assembly strategy, in which poly(ethyleneimine) (PEI) was used to obtain cationic polyelectrolyte-modified ultrathin nanosheets and anchor the Au@AgNPs. The g-C3N4 nanosheets exhibited a strong enrichment ability and the self-assembled Au@AgNPs showed an excellent SERS activity, both of which led to an ultrahigh sensitivity. The hybrids were applied to detect folic acid (FA) with the sensitive detection limit of 2.41 nM. Importantly, after being modified with FA, which targeted cancer cells with folate receptors (FRs), the formed g-C3N4/Au@AgNPs–FA was used as a SERS probe for the on-site monitoring of cancer cells with FA as the Raman reporter molecule.
1. Introduction
SERS has been widely used as a very important analytical technique with impressive sensitivity in a number of applications, such as food safety,1 explosives detection,2 environmental monitoring,3 biomedical research,4 bioimaging5 and so on. Noble metal nanoparticles (Au, Ag) are often chosen for fabricating SERS substrates because of their optical activities by supporting localized surface plasmon resonances (LSPRs).6 SERS could improve the signal intensity of the molecules that are on or near the substrates by orders of magnitude, even with the ability of single molecule detection.7 Compared to individual nanoparticles, the Ag/Au-based composite materials have gradually attracted attention due to their well-defined structures, higher Raman activity, better stability and biocompatibility.8 However, it is still a challenge to fabricate SERS substrates with large enhancement abilities and good reproducibilities, particularly for molecules which have poor affinity to noble metals.In the past few years, ultrathin two-dimensional (2D) layered nanomaterials have attracted tremendous attention due to their excellent electronic and optical properties, biological compatibilities, and high surface areas in contrast to the bulk materials.9 As an analogue of graphite, graphitic-phase carbon nitride (g-C3N4) is the most stable allotrope of carbon nitride.10 The ultrathin g-C3N4 nanosheets have a high surface-to-volume ratio, good biocompatibility and low toxicity, which show great potential applications in Cu2+ detection,11 glucose detection,12 drug delivery13 and bioimaging.14 Furthermore, the ultrathin g-C3N4 nanosheets are considered as a prospective supporting material for metal nanoparticles to form hybrids. Here, a self-assembly strategy was developed to obtain g-C3N4/Au@AgNP hybrids, in which PEI was used to functionalize g-C3N4 nanosheets and anchor the Au@AgNPs.15 The above hybrids could be used as a SERS-active material, in which Au@AgNPs could enhance the Raman scattering while g-C3N4 could concentrate the molecules with high enrichment capacity. The hybrids were used as the SERS substrates to detect R6G with an enhancement factor as high as 3.0 × 1016. Moreover, the as-fabricated substrates could be applied to enhance the Raman signals of folic acid (FA), showing a detection limit as low as 2.41 nM.
FA is a typical cell-targeting agent, which has a high affinity for folate receptors (FRs). FRs are overexpressed on the surface of some human cancer cells and are absent on normal cells, as a result, FRs could be used to distinguish cancer and normal cells.16 Up to now, FA-containing nanomaterials have been developed to target cancer cells owing to FA having a very high affinity to the FRs on cancer cells. Taking into account that fluorescence-based techniques exhibited some disadvantages such as spectral overlap, SERS had extremely high sensitivity and sharp peaks which can be used to distinguish multiple analytes in a mixture. The modification of FA on the nanomaterials occurs mainly in two ways: covalent17 and noncovalent18 binding. In contrast to covalent modification, noncovalent binding has less impact on the materials. With such an effect, the modification of FA via physisorption may provide a reliable, simple and highly efficient route.
In this paper, a facile and efficient SERS probe was prepared and used for cancer diagnosis, which was composed of g-C3N4/Au@AgNP hybrids and FA (g-C3N4/Au@AgNPs–FA). The above-mentioned SERS probe was used to detect the cancer cells, and exhibited very low toxicity. It was mentioned that FA was used both as the Raman reporter molecule and the targeting ligand with the cancer cells. Finally, the excellent SERS and cell-targeting properties of g-C3N4/Au@AgNPs–FA were investigated using HeLa cells which over-express FRs (FR-positive) and A549 cells as the control which express few FRs (FR-negative).
2. Experimental section
2.1 Materials
Sodium citrate (Na3C6H5O7·2H2O, 99.8%), silver nitrate (AgNO3, 99%), chloroauric acid (HAuCl4·4H2O, 99.9%), polyvinyl pyrrolidone (PVP) and melamine were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Poly(ethyleneimine) (PEI), folic acid (FA) and 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich. All of these chemical regents were used without further purification. Ultrapure water (18.2 MΩ cm) used in all reactions was produced using a Millipore water purification system.2.2 Synthesis of the Au@Ag nanoparticles
AuNPs were used as seeds to prepare Au@Ag nanoparticles. Firstly, the AuNPs were obtained by the reduction of HAuCl4 with sodium citrate. Typically, 1.5 mL of 1% trisodium citrate was quickly injected into boiling ultrapure water (100 mL) which contained 2.5 × 10−5 M HAuCl4. Then, the mixture was kept boiling for 30 min. Eventually, the solution became wine red and AuNPs were synthesized. Secondly, 4 mL of 1% trisodium citrate was added into the AuNP solution. Then, 1 mM AgNO3 (17 mL) was added drop by drop into the above mixture. The AgNO3 could be reduced on the surface of the AuNPs to form the Au@AgNPs. Finally, the color of the solution changed from wine red to orange yellow.2.3 Synthesis of the Ag nanoparticles
The AgNPs were synthesized by the reduction of AgNO3 with sodium citrate. In detail, 250 mL of aqueous solution containing 90 mg of AgNO3 was first heated to boiling, then 1% sodium citrate (10 mL) was quickly injected into the above boiling solution. After refluxing for 1 h, the resultant yellow-green colloid was cooled to room temperature.2.4 Synthesis of bulk g-C3N4
The bulk g-C3N4 was obtained from the polymerization of melamine molecules at 600 °C under air conditions, and then kept at that temperature for 2 h.14 The melamine was heated to 600 °C with a constant heating rate of 3 °C min−1, and the same ramp rate was used for the cooling process.2.5 Synthesis of the ultrathin g-C3N4 nanosheets
The ultrathin g-C3N4 nanosheets were prepared using ultrasound treatment of the as-prepared bulk g-C3N4 in water for about 20 h.14 Subsequently, the formed suspension was centrifuged at 6000 rpm to eliminate the unexfoliated g-C3N4 before further use.2.6 Synthesis of the PEI-functionalized ultrathin g-C3N4 nanosheets
In a typical procedure, 60 mg of PVP was added to 100 mL of ultrathin g-C3N4 nanosheet solution, followed by ultrasound treatment for 30 min and stirring for 90 min. The obtained solution was washed two times at 6000 rpm for 20 min to remove the free PVP and then dispersed into 10 mL of ultrapure water. To obtain the PEI-functionalized ultrathin g-C3N4 nanosheets, 7 mL of 1% PEI was mixed well with 40 mL of 0.5 M KCl, then the above PVP-capped ultrathin g-C3N4 nanosheets were added. The final solution was sonicated for 90 min. The obtained solution was washed three times at 6000 rpm for 8 min to remove the free PEI, and then the synthesized PEI/ultrathin g-C3N4 nanosheets were redispersed in 10 mL of ultrapure water.2.7 Synthesis of the ultrathin g-C3N4 nanosheet/Au@Ag nanoparticle hybrids (g-C3N4/Au@AgNPs)
500 μL of the PEI/ultrathin g-C3N4 nanosheets was added into 5 mL of Au@Ag nanoparticle solution under ultrasound before placed the above solution overnight. Finally, the obtained g-C3N4/Au@AgNPs hybrids were washed three times with ultrapure water and redispersed. The ultrathin g-C3N4 nanosheet/AgNPs (g-C3N4/AgNPs) nanocomposites were also obtained similarly.2.8 Synthesis of the ultrathin g-C3N4 nanosheets/Au@Ag nanoparticles–FA (g-C3N4/Au@AgNPs–FA)
The loading of FA on ultrathin g-C3N4 nanosheets/Au@AgNPs was achieved by mixing g-C3N4/Au@AgNPs with FA (1 × 10−4 M) solution overnight. After that, the unbound FA was washed using centrifugal washing. Finally, g-C3N4/Au@AgNPs–FA was redispersed into PBS buffer (pH ∼ 7.4) for further use.2.9 Cell culture and viability measurements
HeLa cells and A549 cells were cultured in high glucose Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA), which was supplemented with 10% (v/v) fetal bovine serum (FBS, Invitrogen), penicillin (100 units per mL), and streptomycin (100 g mL−1) at 37 °C in a humidified incubator (MCO-18AC, Sanyo, Japan) containing 5% CO2. The effect of g-C3N4/Au@AgNPs–FA on cell proliferation was investigated using an MTT assay. Firstly, the HeLa cells were seeded onto a 96-well plate and incubated for 24 h. After that, g-C3N4/Au@AgNPs–FA with a predetermined concentration (0, 75, 150, 300, 450 μg mL−1) was added to the cells. After 24 h of incubation, the medium was changed to MTT solution, and the cells were incubated for another 4 h. Finally, a Bio-Rad ELISA reader was used to measure the viability of the cells at 570 nm. For the control, the cells were incubated in the absence of g-C3N4/Au@AgNPs–FA.2.10 SERS detection of live cancer cells
The HeLa cells and A549 cells were seeded into the sterile glass coverslips of culture Petri dishes and incubated for 24 h. Then, the culture medium was changed to the culture medium containing g-C3N4/Au@AgNPs–FA and incubated for another 2 h at 37 °C. Before measurement, the culture dishes were washed three times to remove the free g-C3N4/Au@AgNPs–FA. The Raman measurements were performed using a 532 nm laser and a 10× objective, with a laser power of 2 mW and the acquisition time of 2 s. The Raman mapping of cells was carried out using DXR Raman microscopy with a 532 nm laser (2 mW) and a 50× objective lens, the accumulation time for each spectrum was 1 s.2.11 Measurements
The UV-vis absorption spectra were recorded using a Shimadzu UV-2550 spectrometer. The ultrathin g-C3N4 nanosheets and Au@AgNPs were characterized using transmission electron microscopy (TEM, JEOL-2010) and field-emission scanning microscopy (FE-SEM, Sirion-200). Atomic force microscopy (AFM) images were obtained using a DI Innova. The Zeta-potential measurements were measured using a Zetasizer 3000 HSA. The Raman measurements were conducted using a Thermo Fisher DXR Raman Microscope equipped with a CCD detector with the excitation wavelength of 532 nm. The XRD was recorded using a MAC Science Co. Ltd MXP 18 AHF X-ray diffractometer.3. Results and discussion
The fabrication procedure of the g-C3N4/Au@AgNP composite structures and their application in the detection of cancer cells are shown in Fig. 1. The Au@AgNPs were prepared using AuNPs with sizes of 30 nm as the “seeds” and ascorbic acid solution as the reductive agent. Subsequently, the silver nitrate solution was added drop by drop under vigorous stirring.1b Since the crystal structures of Au and Ag match very well, the resultant Ag was selectively grown on the surface of the gold particles to form the core–shell Au@AgNPs (∼45 nm in diameter), accompanying an obvious color change from wine red to orange (Fig. S1, ESI†).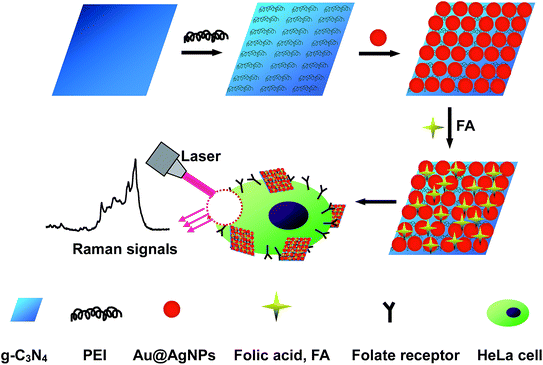 | ||
| Fig. 1 A schematic illustration of the fabrication procedure of g-C3N4/Au@AgNPs–FA as a SERS probe and its application in cancer cell diagnostics. | ||
Benefiting from the unique physical and chemical properties such as a high surface area, remarkable biocompatibility and ease of functionalization, 2D layered nanomaterials have shown great potential in biochemistry and biomedicine.19 The metal-free g-C3N4 is a typical 2D nanomaterial with good biocompatibility and low toxicity. The g-C3N4 bulk materials were obtained from the polymerization of melamine molecules (Fig. S2, ESI†). The ultrathin g-C3N4 nanosheets were obtained by sonicating bulk g-C3N4 in water for about 20 h (Fig. S3, ESI†). As can be seen from Fig. S4,† the IR spectrum of the g-C3N4 nanosheets shows nearly identical absorption bands compared to the bulk g-C3N4, and the Raman spectra of them are also almost the same (Fig. S5, ESI†). The above experimental results confirmed that the intrinsic properties of the ultrathin g-C3N4 nanosheets had not changed compared with the bulk materials.
The zeta potential of the resulting ultrathin g-C3N4 nanosheets was about −35.8 mV, which indicated that they were negatively charged (Table S1, ESI†). On the other hand, the Au@AgNPs were also negatively charged, with a zeta potential of about −42.6 mV. Based on the electrostatic repulsion between the same charge, the ultrathin g-C3N4 nanosheets were very weak for anchoring Au@AgNPs directly onto them. In order to solve the above problems, the ultrathin g-C3N4 was first modified using polyvinylpyrrolidone (PVP). PVP is a nontoxic and biocompatible polymer surfactant, which is often used as a stabilizing agent and dispersing agent in the preparation of nanostructures.20 Then, a positively charged polymer named poly(ethyleneimine) (PEI) was used to modify the ultrathin g-C3N4 nanosheets. PEI is positively charged because there are so many basic amino groups on the polymer chains.1a Finally, the PEI molecules, which modified the ultrathin g-C3N4 nanosheets, can anchor the negatively charged nanoparticles onto the g-C3N4 nanosheets. Finally, FA was attached to g-C3N4/Au@AgNPs via non-covalent binding. FA was used as not only a Raman reporter molecule but also as a targeting molecule to distinguish the cancer cells.
The morphologies of the ultrathin g-C3N4 nanosheets and the Au@AgNP-decorated ultrathin g-C3N4 nanosheets were characterized using transmission electron microscopy (TEM). Fig. 2A shows the typical TEM image of ultrathin g-C3N4 nanosheets. The water suspension of the ultrathin g-C3N4 nanosheets was nearly transparent, and its concentration was estimated to be 0.15 mg mL−1.14 The dynamic light scattering data showed that the dimension of the ultrathin g-C3N4 nanosheets was more than 100 nm, mainly in the 200 nm to 600 nm region (Fig. S6, ESI†). As shown in Fig. 2B–E, all of the Au@AgNPs which had uniform sizes and shapes were confined in the range of the ultrathin g-C3N4 nanosheets. The concentration of the Au@AgNPs was about 0.20 nM based on the concentration of the AuNP seeds (calculated using Beer’s law and the extinction coefficient).1b In order to achieve the optimum SERS activity, the ultrathin g-C3N4 nanosheets loaded with the Au@AgNPs were examined by changing the concentrations of the Au@AgNPs and keeping the identical ultrathin g-C3N4 nanosheets (0.15 mg mL−1). The concentration of the Au@AgNPs was changed from 0.05 to 0.40 nM. Only a few nanoparticles were dispersed on the ultrathin g-C3N4 nanosheets after the 0.05 nM Au@AgNP addition (Fig. 2B). When the concentration of the Au@AgNPs was increased, the nanoparticles loaded on the ultrathin g-C3N4 nanosheets became obviously more intense (Fig. 2C). As the concentration of the Au@AgNPs was increased to 0.20 nM, a uniform, high density Au@AgNP-decorated ultrathin g-C3N4 nanosheet nanocomposite was obtained (Fig. 2D). When the concentration of the Au@AgNPs was increased to 0.40 nM, it was clearly observed that most areas of the nanocomposite were dark in color, which was mainly due to the overlapping of the loaded Au@AgNPs (Fig. 2E). The HRTEM images (Fig. S7, ESI†) and chemical maps of the g-C3N4/Au@AgNPs (Fig. S8, ESI†) were obtained, which further clearly showed the hybrid structure. The g-C3N4/Au@AgNP nanocomposite was also confirmed using XRD, and the diffraction peaks and relative intensity matched with the standard g-C3N4 and Au@AgNP powder diffraction data (Fig. S9, ESI†).
The Au@AgNPs loaded on the surface of the ultrathin g-C3N4 nanosheets were also confirmed by UV-vis spectra. As shown in Fig. 2F, when the Au@AgNPs were loaded on the ultrathin g-C3N4 nanosheets, a new absorption peak corresponding to the Au@AgNP plasmon with a wavelength of 350–550 nm emerged. The line of Fig. 2F from “a” to “e” corresponds to Fig. 2A–E, respectively. As the number of Au@AgNPs loaded on the ultrathin g-C3N4 nanosheets increased, the absorption peaks mentioned above became more and more strong. Once mixed, a distinct color change was observed as the suspension changed from nearly transparent to orange with the increasing concentration of Au@AgNPs.
To further determine the layers of the g-C3N4 nanosheets, atomic force microscopy (AFM) measurements were also obtained. As shown in Fig. 3A, the ultrathin g-C3N4 nanosheets had nearly the same thicknesses. At the same time, Fig. 3B shows that the average height of the g-C3N4 nanosheets randomly measured was about 1.2 nm, which indicated that the ultrathin nanosheets were composed of about three layer forms.13,14 AFM was also used to study the g-C3N4/Au@AgNPs nanocomposite in Fig. 3D. According to the AFM and height images, the changing of the thickness and roughness was monitored. As shown in Fig. 3C, the Au@AgNPs with diameters around 45 nm were loaded on the surface of the g-C3N4 nanosheets. After loading of the Au@AgNPs, it was observed that the roughness of the nanosheets increased and lots of nanoparticles were found. As shown in Fig. 3D, the thickness of the g-C3N4/Au@AgNP hybrid was found to be from 50 to 90 nm, which is significantly thicker than that of the g-C3N4 nanosheets, indicating that the Au@Ag nanoparticles were assembled on g-C3N4, corresponding to the TEM image. Finally, the AFM image showed the formation of the nanocomposites and proved that the loading of the Au@AgNPs was efficient on the platform of the g-C3N4 nanosheets.
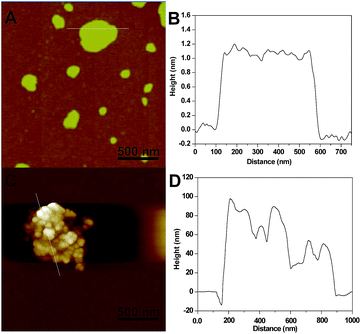 | ||
| Fig. 3 AFM and the corresponding height images of the ultrathin g-C3N4 nanosheets (A and B) and the g-C3N4/Au@AgNP nanocomposites (C and D). | ||
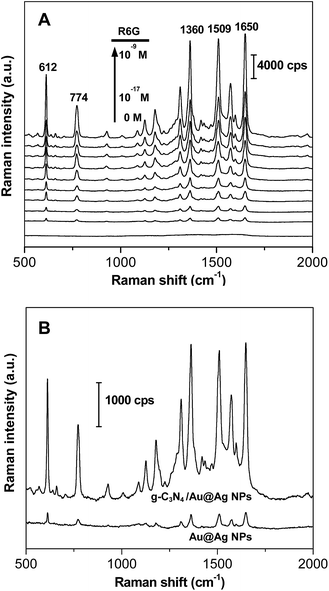
In order to optimize the performance of the g-C3N4/Au@AgNP hybrids as SERS substrates, g-C3N4 nanosheets decorated with different amounts of the Au@AgNPs were synthesized as shown in Fig. 2B–E. To choose the best SERS active substrate, the same conventional Raman-active probe R6G (1.0 × 10−13 M) was used to evaluate the SERS activity of these hybrids with the loading of different densities of the Au@AgNPs (Fig. S10, ESI†). The strong Raman peaks at 612 cm−1, 774 cm−1, 1360 cm−1, 1509 cm−1 and 1650 cm−1 were in good agreement with the previous reports of pure R6G.21 Weak Raman spectra of R6G were detected on the Au@AgNPs, while much stronger signals of R6G were obtained from the g-C3N4/Au@AgNP nanocomposites. Furthermore, the Raman signals of R6G were getting stronger with the increase of the Au@AgNPs decorated on the ultrathin nanosheets. The “hot spots” were formed in the nanoscale gaps among the Au@AgNPs. At the same time, the Au@AgNPs arranged on the ultrathin g-C3N4 nanosheet were not very close together, therefore, there were still many blanks between the Au@AgNPs for the enrichment of analyte molecules in the process of SERS detection. However, when the density of the loaded Au@AgNPs further increased, particles overaggregated which was bad for the SERS signals and the stability of the nanocomposite solution. In our experiments, the g-C3N4/Au@AgNP nanocomposite in Fig. 2D had the best SERS activity and was chosen for all the following tests.
To study the SERS performance of the g-C3N4/Au@AgNPs mentioned above, the SERS spectra of R6G with concentrations ranging from 1.0 × 10−9 to 1.0 × 10−17 M were obtained. As shown in Fig. 4A, the Raman signals were still being observed even when the concentration of R6G decreased to as low as 1.0 × 10−17 M. The SERS intensity of R6G at 1360 cm−1 with different concentrations clearly showed that the g-C3N4/Au@AgNP hybrids had an excellent SERS activity (Fig. S11, ESI†). In addition, the uniformity of the sensitivity of the SERS substrate at every site was very important for the SERS substrates. As shown in Fig. S12,† the Raman spectra of R6G with the concentration of 1.0 × 10−13 M were obtained from ten random sites, suggesting the good uniformity of the g-C3N4/Au@AgNPs (Fig. S12, ESI†). The high SERS performance of the g-C3N4/Au@AgNP nanocomposite could be attributed to the huge surface of the g-C3N4 nanosheets to adsorb more target molecules and the strong electromagnetic enhancement of the Au@Ag NPs. As shown in Fig. 4B, the SERS spectra of R6G (1.0 × 10−13 M) absorbed on the Au@AgNPs were also collected. The hybrids showed an obviously stronger SERS signal than the Au@AgNPs. The SERS experiments confirmed that the ultrathin g-C3N4 nanosheets loaded with the Au@AgNP nanocomposites had superior sensitivity, high uniformity and excellent reproducibility as substrates for Raman applications.
The SERS spectra of FA with a series of concentrations are shown in Fig. 5. The Raman signal of FA could still be observed even when the concentration decreased to as low as 10 nM. The main Raman peaks of FA were consistent with the previous work.15 The intensity of the strongest peak at 1595 cm−1 was used for the quantitative evaluation of the FA level and exhibited a good linear relationship with the concentration ranging from 10 nM to 100 nM (R2 = 0.9976). The limit of detection was determined to be 2.41 nM and was reached based on three standard deviations above the background. Therefore, strong SERS signals of FA were obtained from the g-C3N4/Au@AgNPs compared to the Au@AgNPs, leading to the ultrasensitive detection of FA (Fig. S13, ESI†). In order to obtain a better understanding of the hybrids, g-C3N4 nanosheet/AgNP (g-C3N4/AgNPs) nanocomposites were also prepared similarly. Taking R6G for example, the g-C3N4/Au@AgNPs exhibited an excellent Raman activity that was 3 orders of magnitude higher than the corresponding g-C3N4/AgNPs (Fig. S14, ESI†). The g-C3N4/Au@AgNP nanocomposites owning high SERS activity, stability and water solubility endowed them as a promising material for bioimaging applications.
In this work, the FA was used as both the Raman probe molecule and the targeting ligand for cancer cells with FRs. FA was attached to the surface of the g-C3N4/Au@AgNPs via physisorption, and the non-covalent interaction between them was attributed to π–π* stacking.22 Based on the above, the g-C3N4 nanosheets were used to enrich the FA molecules, at the same time the Au@AgNPs loaded on the nanosheets enhanced the Raman signal of FA. The characteristic Raman signals of FA could be used to identify FA on certain cancer cells by using g-C3N4/Au@AgNPs–FA as the diagnostic probe material.
The modification of FA with the g-C3N4/Au@AgNPs through physisorption was confirmed by the new absorption peaks at 280 and 365 nm corresponding to the standard FA (Fig. S15, ESI†). The cytotoxicity of g-C3N4/Au@AgNPs–FA was investigated using an MTT assay which has been described as a suitable method for the detection of nanoparticle toxicity. As can be seen from Fig. S16,† very little loss of cell viability was observed even with the concentration of incubated g-C3N4/Au@AgNPs–FA as high as 300 μg mL−1, suggesting the excellent biocompatibility and nontoxicity of the hybrids. In addition, it is worth mentioning that the concentration of g-C3N4/Au@AgNPs–FA used in the bioimaging experiment was much lower, only 150 μg mL−1.
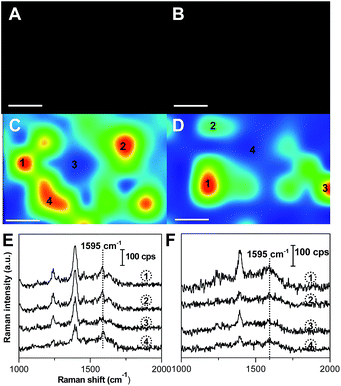
To investigate the targeting ability of g-C3N4/Au@AgNPs–FA, HeLa cells were used as the model cancer cells because they over-express FRs (FR-positive), and A549 cells were selected as the control which express few FRs (FR-negative). The HeLa and A549 cells cultured in Dulbecco’s modified Eagle’s medium (DMEM) were incubated with g-C3N4/Au@AgNPs–FA for 2 h and washed sufficiently with phosphate-buffered saline (PBS) before the experiments. The dark-field images indicated that a large number of the g-C3N4/Au@AgNPs–FA nanocomposites had attached to the FRs after 2 h of incubation (Fig. 6A and C). However, only a small amount of nanocomposites had attached on the A549 cells, indicating the specific targeting of g-C3N4/Au@AgNPs–FA to FR-positive cancer cells. The SERS imagings of HeLa and A549 cells with FA at 1595 cm−1 shown in Fig. 6C and D, which clearly displayed that HeLa cells incubated with g-C3N4/Au@AgNPs–FA exhibited significantly enhanced Raman signals of FA and thus more distinguishable Raman images. Fig. 6E and F describe the corresponding Raman at the indicated sites. It was clear that the SERS spectra of FA was observed and was not overwhelmed by the large background, but the signals of FA were too weak to be distinguished after incubation with the FR-negative A549 cells. The above experiments clearly demonstrated that our SERS probe was able to distinguish cancer cells that over-expressed FRs from the cells that expressed few FRs.
4. Conclusions
In conclusion, we reported the synthesis of a functional g-C3N4/Au@AgNPs–FA hybrid material with good biocompatibility and targeting ability, and its application in the Raman detection of cancer cells. The obtained g-C3N4/Au@AgNPs showed tremendous enhancement of the Raman signals of the absorbed FA, which has the ability to target FR-positive cancer cells and be used as a Raman reporter. The SERS signals of the FA molecules on the FR-positive cancer cells were successfully detected with 532 nm laser excitation. The above experimental results indicate that the g-C3N4/Au@AgNPs–FA does not only have a good targeting capability of live cancer cells but also has great potential as a new Raman probe for cancer diagnosis.Acknowledgements
This work was supported by the National Science Foundation of China (No. 21371174, 21175137, 21375131, 61205152).Notes and references
- (a) L. L. Zhang, C. L. Jiang and Z. P. Zhang, Nanoscale, 2013, 5, 3773–3779 RSC; (b) B. H. Liu, G. M. Han, Z. P. Zhang, R. Y. Liu, C. L. Jiang, S. H. Wang and M. Y. Han, Anal. Chem., 2012, 84, 255–261 CrossRef CAS PubMed.
- (a) J. P. Wang, L. Yang, B. H. Liu, H. H. Jiang, R. Y. Liu, J. W. Yang, G. M. Han, Q. S. Mei and Z. P. Zhang, Anal. Chem., 2014, 86, 3338–3345 CrossRef CAS PubMed; (b) H. Ko and V. V. Tsukruk, Small, 2008, 4, 1980–1984 CrossRef CAS PubMed.
- S. J. Lee and M. Moskovits, Nano Lett., 2011, 11, 145–150 CrossRef CAS PubMed.
- (a) Z. X. Chen, J. J. Li, X. Q. Chen, J. T. Cao, J. R. Zhang, Q. H. Min and J. J. Zhu, J. Am. Chem. Soc., 2015, 137, 1903–1908 CrossRef CAS PubMed; (b) S. R. Panikkanvalappil, S. M. Hira, M. A. Mahmoud and M. A. El-Sayed, J. Am. Chem. Soc., 2014, 136, 15961–15968 CrossRef CAS PubMed.
- (a) C. L. Zavaleta, B. R. Smith, I. Walton, W. Doering, G. Davis, B. Shojaei, M. J. Natan and S. S. Gambhir, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13511–13516 CrossRef CAS PubMed; (b) V. Amendola, S. Scaramuzza, L. Litti, M. Meneghetti, G. Zuccolotto, A. Rosato, E. Nicolato, P. Marzola, G. Fracasso, C. Anselmi, M. Pinto and M. Colombatti, Small, 2014, 10, 3823–3823 CrossRef CAS PubMed; (c) J. Huang, C. Zong, H. Shen, M. Liu, B. A. Chen, B. Ren and Z. J. Zhang, Small, 2012, 8, 2577–2584 CrossRef CAS PubMed.
- (a) S. Schlucker, Angew. Chem., Int. Ed., 2014, 53, 4756–4795 CrossRef PubMed; (b) W. E. Smith, Chem. Soc. Rev., 2008, 37, 955–964 RSC.
- (a) K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan, R. Dasari and M. S. Feld, Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef CAS; (b) S. M. Nie and S. R. Emery, Science, 1997, 275, 1102–1106 CrossRef CAS PubMed.
- (a) Z. Y. Wang, S. F. Zong, W. Li, C. L. Wang, S. H. Xu, H. Chen and Y. P. Cui, J. Am. Chem. Soc., 2012, 134, 2993–3000 CrossRef CAS PubMed; (b) T. X. Yang, X. Y. Guo, H. Wang, S. Y. Fu, J. Yu, Y. Wen and H. F. Yang, Small, 2014, 10, 1325–1331 CrossRef CAS PubMed; (c) Z. L. Song, Z. Chen, X. Bian, L. Y. Zhou, D. Ding, H. Liang, Y. X. Zou, S. S. Wang, L. Chen, C. Yang, X. B. Zhang and W. H. Tan, J. Am. Chem. Soc., 2014, 136, 13558–13561 CrossRef CAS PubMed.
- (a) K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451–10453 CrossRef CAS PubMed; (b) K. P. Loh, Q. L. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 CrossRef CAS PubMed; (c) J. Q. Tian, Q. Liu, A. M. Asiri, K. A. Alamry and X. P. Sun, ChemSusChem, 2014, 7, 2125–2130 CrossRef CAS PubMed; (d) N. Y. Cheng, J. Q. Tian, Q. Liu, C. J. Ge, A. H. Qusti, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6815–6819 CrossRef CAS PubMed.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- (a) C. M. Cheng, Y. Huang, X. Q. Tian, B. Z. Zheng, Y. Li, H. Y. Yuan, D. Xiao, S. P. Xie and M. M. F. Choi, Anal. Chem., 2012, 84, 4754–4759 CrossRef CAS PubMed; (b) N. Y. Cheng, P. Jiang, Q. Liu, J. Q. Tian, A. M. Asiri and X. P. Sun, Analyst, 2014, 139, 5065–5068 RSC; (c) J. Q. Tian, Q. Liu, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Anal. Chem., 2013, 85, 5595–5599 CrossRef CAS PubMed.
- (a) X. R. Du, G. J. Zou, Z. H. Wang and X. L. Wang, Nanoscale, 2015, 7, 8701–8706 RSC; (b) J. Q. Tian, Q. Liu, A. M. Asiri, A. H. Qusti, A. O. Al-Youbi and X. P. Sun, Nanoscale, 2013, 5, 11604–11609 RSC; (c) J. Q. Tian, Q. Liu, C. J. Ge, Z. C. Xing, A. M. Asiri, A. O. Al-Youbi and X. P. Sun, Nanoscale, 2013, 5, 8921–8924 RSC.
- L. S. Lin, Z. X. Cong, J. Li, K. M. Ke, S. S. Guo, H. H. Yang and G. N. Chen, J. Mater. Chem. B, 2014, 2, 1031–1037 RSC.
- X. D. Zhang, X. Xie, H. Wang, J. J. Zhang, B. C. Pan and Y. Xie, J. Am. Chem. Soc., 2013, 135, 18–21 CrossRef CAS PubMed.
- W. Ren, Y. X. Fang and E. K. Wang, ACS Nano, 2011, 5, 6425–6433 CrossRef CAS PubMed.
- (a) H. F. Dong, J. P. Lei, H. X. Ju, F. Zhi, H. Wang, W. J. Guo, Z. Zhu and F. Yan, Angew. Chem., Int. Ed., 2012, 51, 4607–4612 CrossRef CAS PubMed; (b) D. Feng, Y. C. Song, W. Shi, X. H. Li and H. M. Ma, Anal. Chem., 2013, 85, 6530–6535 CrossRef CAS PubMed.
- D. J. Bharali, D. W. Lucey, H. Jayakumar, H. E. Pudavar and P. N. Prasad, J. Am. Chem. Soc., 2005, 127, 11364–11371 CrossRef CAS PubMed.
- (a) J. J. Castillo, T. Rindzevicius, L. V. Novoa, W. E. Svendsen, N. Rozlosnik, A. Boisen, P. Escobar, F. Martinez and J. Castillo-Leon, J. Mater. Chem. B, 2013, 1, 1475–1481 RSC; (b) C. F. Hu, Y. L. Liu, J. L. Qin, G. T. Nie, B. F. Lei, Y. Xiao, M. T. Zheng and J. H. Rong, ACS Appl. Mater. Interfaces, 2013, 5, 4760–4768 CrossRef CAS PubMed.
- G. H. Yang, C. Z. Zhu, D. Du, J. J. Zhu and Y. H. Lin, Nanoscale, 2015, 7, 14217–14231 RSC.
- (a) X. C. Qin, Z. Y. Guo, Z. M. Liu, W. Zhang, M. M. Wan and B. W. Yang, J. Photochem. Photobiol., B, 2013, 120, 156–162 CrossRef CAS PubMed; (b) A. S. Wajid, S. Das, F. Irin, H. S. T. Ahmed, J. L. Shelburne, D. Parviz, R. J. Fullerton, A. F. Jankowski, R. C. Hedden and M. J. Green, Carbon, 2012, 50, 2065–2065 CrossRef CAS PubMed.
- G. F. Li, H. Li, Y. J. Mo, X. J. Huang and L. Q. Chen, Chem. Phys. Lett., 2000, 330, 249–254 CrossRef CAS.
- M. C. Rong, L. P. Lin, X. H. Song, T. T. Zhao, Y. X. Zhong, J. W. Yan, Y. R. Wang and X. Chen, Anal. Chem., 2015, 87, 1288–1296 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16558j |
| This journal is © The Royal Society of Chemistry 2015 |