A surfactant-assisted high energy ball milling technique to produce colloidal nanoparticles and nanocrystalline flakes in Mn–Al alloys
Abstract
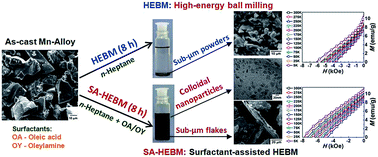
We herein exploit the advantages of surfactant assisted-high energy ball milling (SA-HEBM) for the processing of Mn–Al alloy. In this method, a combination of two surfactants, such as oleic acid and oleylamine, was used along with a solvent, n-heptane, during milling. The use of the SA-HEBM process yielded two different products: a sediment powder consisting of sub-micron sized Mn–Al flakes and a suspension in the milling medium (colloid) containing Mn–Al nanoparticles. The colloid consisted of Mn–Al nanocrystalline particles (5–16 nm) having a lamellar morphology with an average thickness of 1.5 nm and length of 16 nm. Magnetic measurements of these colloidal nanoparticles demonstrated an almost non-magnetic behavior at 300 K. The Mn–Al sediment powders obtained with the SA-HEBM process at regular milling time intervals were investigated for their structural and magnetic performance and also compared with the corresponding powders obtained from the conventional HEBM process. The phase composition of Mn–Al powders processed by both HEBM and SA-HEBM is quite similar and they adhere to the phase constituents of parent alloy: τ-, β-phases with some traces of ε-phase. Nevertheless, the estimated magnetic parameters such as saturation magnetization and coercivity for the SA-HEBM powders exhibited improved values, as compared to the HEBM powders. A maximum coercivity of 4.88 kOe was obtained for the 8 h milled SA-HEBM powder and this value is 23% higher than that of HEBM processed powder. The results were explained based on the effect of surfactants on the morphology of the milled powder.

 Please wait while we load your content...
Please wait while we load your content...