Utilizing an aggregate forming microenvironment sensitive coumarin–cholesterol conjugate as a sensor of pluronic organization and micro-polarity†
Abstract
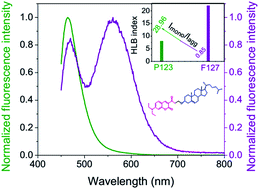
A new fluorescent cholesterol molecular probe, 3-acetyl-7-(diethylamino)-2H-chromen-2-one attached cholesterol (Cum–Chl), has recently been introduced as a reporter of micro-heterogeneous media. The H-aggregate forming ability of Cum–Chl provides a useful fluorescence parameter, Imonomer/Iaggregate to understand the micro-polarity of anisotropic media. Pluronics are surfactant based polymeric anisotropic media having important applications in therapeutics. Being a sensitive indicator of pluronics micro-polarity Imonomer/Iaggregate can help in selecting pluronics for medicinal purposes. Additionally, temperature and concentration induced sol–gel transition of pluronics (P123, F127) have been successfully investigated by Cum–Chl using its Imonomer/Iaggregate value, along with other conventional fluorescence parameters. Finally, this molecular probe, Cum–Chl emerges as a good sensor of progressive polymeric association and micro-polarity of pluronics.

 Please wait while we load your content...
Please wait while we load your content...