Polyaniline/CaCu3Ti4O12 nanofiber composite with a synergistic effect on visible light photocatalysis
H. S. Kushwahaa,
P. Thomasb and
Rahul Vaish*a
aSchool of Engineering, Indian Institute of Technology Mandi, Himachal Pradesh, India. E-mail: rahul@iitmandi.ac.in; Fax: +91-1905-237945; Tel: +91-1905-237921
bDielectric Materials Division, Central Power Research Institute, Bangalore, India
First published on 30th September 2015
Abstract
CaCu3Ti4O12 (CCTO)–polyaniline (PANI) nanofiber composites with different ratios of PANI/CCTO were successfully prepared by in situ polymerization and were shown to be active photocatalysts under irradiation with visible light. The as-synthesized samples were characterized by XRD, SEM, TEM, FTIR, UV-visible spectrophotometry and TGA/DSC. Significant adsorption was observed for anionic dyes because the negatively charged groups or the electron-rich groups of these dyes interacted chemically with the positively charged backbone of PANI. Photocatalytic experiments showed that PANI/CCTO nanofibers with a weight ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 showed the highest photocatalytic activity for the degradation of Congo red and methyl orange under visible light irradiation. The synergistic effect in the PANI/CCTO nanofiber composites results in a reduction of the photoinduced electron–hole recombination rate, giving a high photocatalytic activity for the degradation of the dyes under visible light irradiation. The intense photocatalytic activity can be attributed to the photogenerated electrons in PANI, which can directly transfer to the LUMO of CCTO, and the photogenerated holes in the HOMO of CCTO, which can migrate to the valance band of PANI, efficiently inhibiting direct recombination of the charge carriers.
3 showed the highest photocatalytic activity for the degradation of Congo red and methyl orange under visible light irradiation. The synergistic effect in the PANI/CCTO nanofiber composites results in a reduction of the photoinduced electron–hole recombination rate, giving a high photocatalytic activity for the degradation of the dyes under visible light irradiation. The intense photocatalytic activity can be attributed to the photogenerated electrons in PANI, which can directly transfer to the LUMO of CCTO, and the photogenerated holes in the HOMO of CCTO, which can migrate to the valance band of PANI, efficiently inhibiting direct recombination of the charge carriers.
Introduction
Water pollution has a direct impact on both the balance of nature and human health.1 The availability of fresh water for drinking and agriculture has decreased over recent years as a result of the pollution of natural water bodies.2 Organic dyes are extensively used in the textile, paper production, leather tanning and food technology industries, and in hair colorings.3 Most of these dyes are toxic to aquatic organisms and many have carcinogenic effects on humans. The rapid increase in the production and application of synthetic dyes has resulted in severe water pollution and serious risks to health. The release of large amounts of organic dyes into the environment is a challenge for environmental scientists in terms of increased public concern and the need to meet the requirements of environmental legislation.4–6 Different techniques such as adsorption, oxidation, reduction, electrochemical methods and membrane filtration have been used to remove organic dyes from water and waste water to reduce harmful effects on the environment.7,8Photocatalysis is a green technology and has been widely applied to air purification and the remediation of waste waters.9,10 Since the success of Fujishima and Honda in 1972,11 TiO2 has become the most widely studied semiconductor in the photocatalysis field. However, TiO2 can only absorb 4% of the sunlight spectrum due to its large band gap of 3.2 eV. Therefore the development of highly efficient photocatalysts for waste water treatment that are active in visible light has become an important area of research.12,13 To develop efficient photocatalysts that are active under visible light irradiation, and have a slow rate of recombination of charge carriers and high stability, current research is mainly focused on the modification of the surface or bulk properties of semiconductor materials. This is achieved by doping the semiconductors with nonmetal atoms, combining them with noble metals, using narrow band gap semiconductors, and dye sensitization.14,15 An effective strategy is to modify the surface of the semiconductor photocatalyst with conjugated materials that absorb visible light. Conjugated materials – such as carbon nanotubes, fullerene C60, graphene and polymers with an extended π-conjugated electron system – are good hole transporters and efficient electron donors.14,16,17 These conjugated materials can harvest visible light for the generation of photoelectrons and then inject the excited electrons into the conduction band of the semiconductor photocatalyst.
Over the past decade, polyaniline (PANI) has received much attention as a result of its good stability, corrosion protection, nontoxicity, facile and low-cost synthesis, and high intrinsic redox properties.18,19 Some efforts have been made to use PANI-based nanocomposites under irradiation with visible light. Table 1 shows the results of using selected PANI-based photocatalysts for the degradation of synthetic dyes under irradiation with visible light. It has been reported that PANI can be used for the selective removal of anionic dyes such as Congo Red (CR) and methyl orange (MO) from aqueous solutions and that the interaction between the negatively charged anion of the dye and the positively charged PANI backbone is responsible for the adsorption of the anionic dye by PANI in aqueous solution.20,21 It is well known that PANI, as a conducting polymer with an extended π-conjugated electron system, has shown great potential as a result of its high absorption coefficient in the visible range and the high mobility of its charge carriers.22 PANI, in both its undoped or partially doped state, is an electron donor when photoexcited and has also been reported to be a good hole conductor capable of carrying a current of several milliamperes. A synergistic effect between semiconductors and PANI results in a high efficiency of separation of the photogenerated electron–hole pairs.19,23 This results in enhanced photoactivity and protects the semiconductor particles from photocorrosion.
| No. | Photocatalyst | Pollutant | Kinetic constant (k) | Reference |
|---|---|---|---|---|
| 1 | P25 | MO | 0.011 h−1 | 21 |
| 2 | P25 | CR | 0.012 h−1 | 21 |
| 3 | TiO2–PANI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
MO | 0.031 h−1 | 21 |
| 4 | TiO2–PANI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
CR | 0.037 h−1 | 21 |
| 5 | CoFe2O4–PANI | MO | 0.18 h−1 | 30 |
| 6 | TiO2–PANI | MO | 0.53 h−1 | 20 |
| 7 | TiO2–PANI | MB | 0.003 min−1 | 31 |
| 8 | TiO2–PANI | RhB | 0.019 min−1 | 31 |
| 9 | BiVO4–3% PANI | RhB | 0.01 min−1 | 32 |
| 10 | PANI–Bi12TiO20 | RhB | 0.01 min−1 | 33 |
| 11 | PANI–ZnO | MB | 0.02 min−1 | 34 |
| 12 | PANI–CoFe2O4 | MO | 0.03 min−1 | 35 |
| 13 | PANI/CCTO | MO | 0.056 min−1 | Present work |
| 14 | PANI/CCTO | CR | 0.064 min−1 | Present work |
ABO3 perovskite and layered structures have great potential in visible light photocatalysis and are considered to be third-generation photocatalysts. The band structure of these materials can be tailored by substitution of the A and B sites.24 Double perovskite structures such as A2InNbO6 and A3CoNb2O9 have shown potential in the splitting of water under visible light.25,26 The photocatalytic performance of a catalyst mainly depends on: (i) its light absorption properties; (ii) the rates of reduction and oxidation on the surface of the catalyst by the electrons and holes; and (iii) the electron–hole recombination rate. CaCu3Ti4O12 (CCTO) is a combination of the well-known highly UV-active photocatalyst TiO2 and CuO and absorbs visible light.27 CCTO is a cubic double perovskite with Cu2+ and Ca2+ on the A site and Ti4+ on the B site. It has been extensively studied for energy storage and microelectronics applications as a result of its high dielectric constant.28,29
We report here the controlled synthesis of a PANI/CCTO nanofiber photocatalyst prepared via in situ oxidative polymerization. It was found that the photocatalytic efficiency of the nanofibers was affected by the molar ratio of CCTO and PANI. The PANI/CCTO nanofibers showed highly enhanced photodegradation of CR and MO under visible light irradiation. In particular, the photocatalytic properties of the as-synthesized PANI/CCTO nanofibers were studied. Synergistic effects in the combination of CCTO and PANI were investigated and showed that the composite has high photocatalytic activity for the degradation of dyes under visible light irradiation.
Experimental
Materials and characterization
The following chemicals and reagents were used for the preparation of the PANI–CCTO composites: TiCl4 (99.98%) (Merck, Germany), CaCo3 (BDH; A.R. grade), CuCl3 (Fluka, Proanalyse grade), oxalic acid (S.D. Fine Chemicals, analytical grade), ethanol and acetone (Nice, India; 99.5% pure), aniline monomer as received (Merck, India), ammonium persulfate (APS), HCl (S.D. Fine Chemicals) and sodium carboxymethyl cellulose.X-ray powder diffraction studies were carried out using an X'Pert-Pro diffractometer (Rigaku) using Cu Kα1 radiation (λ = 0.154056 nm) over a wide range of 2θ (5 ≤ 2θ ≤ 85) with a 0.017° step size. Scanning electron microscopy (SEM) (Nova NanoSEM, FEI-Technai) was used to study the surface morphology of the composites. Transmission electron microscopy (TEM) was carried out using an FEI-Technai instrument (G-F30, Hillsboro, OR, USA). Fourier-transform infrared (FTIR) spectrometry was carried out using a Perkin-Elmer FTIR spectrophotometer and the KBr disk technique. UV-visible diffuse reflectance spectra were recorded using a Perkin-Elmer UV-visible spectrophotometer. Thermal gravimetric analysis (TGA) was carried out using a TA Instruments (UK) TGA Q500 system with alumina as the reference material. The experiments were carried out at a heating rate of 10 °C min−1 in a flowing N2 atmosphere (flow rate 50 cm3 min−1). The photocurrent responses were measured on an electrochemical system (Autolab Metrohm). A 500 W Xe lamp was used for visible light irradiation (λ > 420 nm). A 420 nm cut-off filter was used to completely remove any radiation at wavelengths < 420 nm.
Preparation of CaCu3Ti4O12 nanoparticles
CaCu3Ti4O12 nanoparticles were synthesized using an oxalate precursor. Titanium oxide gel was prepared from aqueous TiOCl2 (0.05 M) by adding NH4OH (aq.) (at 25 °C) until the pH reached 8.0 and NH4Cl was washed from the filter funnel. The as-synthesized gel was added to 0.4 or 0.8 mol of oxalic acid (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of Ti
2 ratio of Ti![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2O42−) and heated at 40 °C. Calcium carbonate was added to obtain a clear solution. An aqueous solution containing titanyl oxalic acid together with calcium titanyl oxalate remained clear without the formation of any precipitate. This solution was cooled to 10 °C and then cupric chloride dissolved in acetone was added with water (80/20) and the mixture was stirred continuously. A thick precipitate separated out on the addition of more acetone. The precipitate was filtered, washed several times with acetone to remove chloride, and then dried in air. The precursor was isothermally heated around 700 °C to obtain nanoparticles of phase-pure calcium copper titanate, CaCu3Ti4O12, which were confirmed by X-ray diffraction (XRD).
C2O42−) and heated at 40 °C. Calcium carbonate was added to obtain a clear solution. An aqueous solution containing titanyl oxalic acid together with calcium titanyl oxalate remained clear without the formation of any precipitate. This solution was cooled to 10 °C and then cupric chloride dissolved in acetone was added with water (80/20) and the mixture was stirred continuously. A thick precipitate separated out on the addition of more acetone. The precipitate was filtered, washed several times with acetone to remove chloride, and then dried in air. The precursor was isothermally heated around 700 °C to obtain nanoparticles of phase-pure calcium copper titanate, CaCu3Ti4O12, which were confirmed by X-ray diffraction (XRD).
Preparation of PANI–CaCu3Ti4O12 nanofibers
The PANI–CaCu3Ti4O12 nanocrystal composites were prepared by in situ polymerization in HCl. As a first step, CCTO nanoparticles were added to sodium carboxyl methyl cellulose, which acts as a dispersant, in the ratio 1.5 sodium carboxyl methyl cellulose to 100 CCTO in 100 ml of diluted HCl (pH 1–2). The suspension was then ultrasonicated to avoid agglomeration and to wet the nanoparticles with sodium carboxyl methyl cellulose. A 2 g mass of aniline monomer was then added to this suspension and stirred for 1–2 h. A 7.35 g mass of APS in 30 ml of 1 M HCl solution was added dropwise to the mixture and stirred for 12 h at room temperature. The dark green solid mass obtained was washed several times with dilute HCl and ethanol. The solid mass was filtered and dried in a vacuum oven at 80–100 °C for 24 h. Composites with varying concentrations of CCTO (10, 30 and 50%) by weight were fabricated.The PANI characterized in this investigation has also been prepared by a similar method without the addition of CCTO nanoparticles.
Adsorption studies
A 50 mg mass of CCTO, PANI or PANI/CCTO nanofibers was added to a flask containing 100 mL of dye solution at an initial concentration of 100 mg L−1 and stirring magnetically for 2 h in the dark. Sample aliquots were collected at different time intervals and the concentration of dye in the aliquots was determined using a UV-visible spectrophotometer (Shimadzu UV-2550). The dye concentrations were calibrated using the Beer–Lambert law at characteristic λmax values for CR and MO of 497 and 460 nm, respectively.Photocatalytic studies
CR and MO were selected as representative pollutants of water to determine the efficiency of the as-prepared PANI/CCTO nanofiber photocatalyst under visible light irradiation. In a typical procedure, 0.05 g of photocatalyst was dispersed in 20 mL of the aqueous solutions of these organic dyes (pH = 7). A 500 W Xe lamp equipped with a 420 nm cut-off filter served as the light source for photocatalysis. To achieve a good dispersion and establish an adsorption–desorption equilibrium between the dye and the photocatalyst, the suspensions were stirred continuously for 120 min at 900 rpm in the dark prior to the photocatalysis process. Cold water was circulated around the reaction vessel during light exposure to maintain the reaction at room temperature. During the photocatalysis, the sample aliquots were collected at different time intervals and the photocatalyst powder was removed by centrifugation at 7000 rpm. The organic dye concentration was measured before and after degradation by UV-visible spectrophotometry. The active species generated in the photocatalytic system could be measured by trapping with t-butyl alcohol (t-BuOH) and disodium ethylene diamine tetra acetate dihydrate (EDTA-2Na).Results and discussion
Fig. 1 shows the X-ray powder diffraction patterns obtained for pure PANI, pure CCTO and PANI/CCTO composites with different weight fractions of CCTO. The diffraction peak at 2θ = 25° (200) (Fig. 1a) is typical of PANI.36 The peaks corresponding to 2θ = 43.8, 49 and 50.9° are attributed to the nanocrystalline structure of PANI.37 Fig. 1b shows the XRD pattern obtained for CCTO nanoparticles and this compares well with the ICDD data (01-075-1149).28 The XRD patterns obtained for the synthesized PANI/CCTO composites are shown in Fig. 1c–e. As the CCTO content increased in the PANI, the peaks corresponding to CCTO dominated.38 The peak at 25° (200) corresponding to PANI (Fig. 1c) for the composite 90% PANI + 10% CCTO is less intense than that of pure PANI (Fig. 1a). The intensity (Fig. 1d) decreased further for the composite corresponding to 70% PANI + 30% CCTO. | ||
| Fig. 1 XRD patterns for (a) PANI, (b) CCTO, (c) 90% PANI + 10% CCTO, (d) 70% PANI + 30% CCTO, and (e) 50% PANI + 50% CCTO. | ||
The SEM micrograph obtained for the PANI/CCTO nanofibers is shown in Fig. 2. The micrograph shows the agglomerated PANI, which, on careful examination at high magnification, revealed the existence of nanofibers in the polymer. Fig. 3a shows the bright field TEM image of PANI illustrating the nanofibrous morphology. It can be observed that the dimensions of the PANI nanofibers ranged from 150 to 200 nm in width, although the aspect ratio could not be specified as there was some agglomeration and non-uniform thickness associated with the fibers. The dimensions of the PANI and CCTO fibers were determined and it was observed (Fig. 3b) that the width of the PANI fibers was about 300 nm and the CCTO crystallites embedded in the PANI were in the range 30–50 nm. Fig. 3c shows a magnified image of the CCTO crystallites embedded in the PANI matrix. As Fig. 3c was obtained at a higher magnification, the fibers can be observed in more detail.
Fig. 4 shows the FTIR spectra recorded for pure PANI, nanocrystalline CCTO and the PANI/CCTO nanofibers. The characteristic peaks of PANI were observed around 1567, 1484, 1301, 1242 and 1118 cm−1. The absorption peaks at 1567 and 1484 cm−1 represent the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching of the quinoid and benzenoid rings. The peaks at 1301 and 1242 cm−1 are attributed to the C–N stretching modes of the benzenoid rings, whereas the band at 1118 cm−1 is the result of an in-plane bending vibration of C–H.39 The peak at 801 cm−1 is attributed to the out-of-plane deformation of C–H in the p-disubstituted benzene ring.40 The IR spectrum recorded for the CCTO nanoparticles is depicted in Fig. 4e. The absorption bands in the region 380–700 cm−1 result from the mixed vibrations of the CuO4 and TiO6 groups in the CCTO structure. The IR spectra recorded for the composites (Fig. 4a–c) show similar spectra to that of pure PANI, unlike in the XRD profiles where characteristics of both PANI and CCTO were observed.41
C stretching of the quinoid and benzenoid rings. The peaks at 1301 and 1242 cm−1 are attributed to the C–N stretching modes of the benzenoid rings, whereas the band at 1118 cm−1 is the result of an in-plane bending vibration of C–H.39 The peak at 801 cm−1 is attributed to the out-of-plane deformation of C–H in the p-disubstituted benzene ring.40 The IR spectrum recorded for the CCTO nanoparticles is depicted in Fig. 4e. The absorption bands in the region 380–700 cm−1 result from the mixed vibrations of the CuO4 and TiO6 groups in the CCTO structure. The IR spectra recorded for the composites (Fig. 4a–c) show similar spectra to that of pure PANI, unlike in the XRD profiles where characteristics of both PANI and CCTO were observed.41
 | ||
| Fig. 4 FTIR spectra of (a) 50% PANI + 50% CCTO, (b) 70% PANI + 30% CCTO, (c) 90% PANI + 10% CCTO, (d) PANI, and (e) CCTO. | ||
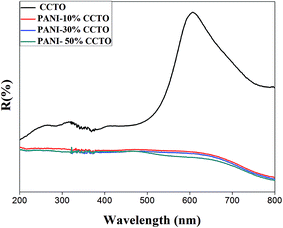
The optical absorption properties of photocatalysts are of great significance in the photocatalysis process. Fig. 5 shows the UV-visible diffuse reflectance spectra of CCTO and PANI/CCTO composites. The CCTO powder has two broad bands, one around 220–550 nm and another around 700 nm. The first band can be resolved by fitting the curve into three components with maxima around 330, 370 and 470 nm. The intensity of these components is much higher than that of the 700 nm band. The brown color of the samples can be explained in terms of a higher absorbance in the blue region than in the red region. The distorted tetrahedral (squashed tetrahedra) stereochemistry may account for the brown-colored Cu(II) compounds. Considering the hole formalism for 3d9 ions, there are three possible transitions: (i) dx2−y2 → dxy; (ii) dx2−y2 → dz2; and (iii) dx2−y2 → dzx, yz. The band around 700 nm corresponds to transition (i), whereas that around 470–550 nm corresponds to transition (ii). The absorption due to transition (iii) of Cu(II) is at a maximum around 370 nm as a result of the overlap with the charge-transfer band, which tails off into the blue region. The charge-transfer band also arises from the O 2p6 to Ti 3d0 excitation of TiO6, which is at a maximum around 330 nm. In CCTO, the hybridization of the O 2p and Cu 3d orbitals is strong, so there is a higher covalent character for the Cu–O bond than for the Ti–O bond. Thus the valence band consists of O 2p and Cu 3d states with a lower contribution from the t2g state of Ti 3d0, whereas the conduction band mostly consists of the Ti 3d states. PANI shows characteristic peaks at 290, 434 and 715 nm, which are attributed to top–p*, polaron–p* and p–polaron transitions in the PANI molecules, respectively. A higher absorbance was observed in the PANI/CCTO nanofibers as a result of the strong absorbance of PANI. The absorption bands of CCTO were not observed in the composite nanofibers, perhaps as a result of the enhancement in absorption intensity by the surrounding PANI.
Thermogravimetric analysis (TGA) of pure PANI and the PANI/CCTO nanofibers was carried out to investigate the thermal stability (Fig. 6). PANI exhibited three typical steps of weight loss behavior.42 In the first step, weight loss occurred around 110 °C as a result of the loss of absorbed water. The second step around 285 °C represents the coevolution of water, acid or a phase transition. The third step was not clearly seen, however, PANI is degraded above 285 °C. The observed weight loss for PANI at 590 °C is around 39% and the weight loss decreased as the weight fraction of the CCTO in the PANI–CCTO composite increased. This behavior is similar to that reported previously for PANI-based composites.41,42 These results indicate that the PANI/CCTO nanofibers have a better thermal stability than pure PANI.
 | ||
| Fig. 6 TGA curves for (a) PANI, (b) 90% PANI + 10% CCTO, (c) 70% PANI + 30% CCTO, and (d) 50% PANI + 50% CCTO. | ||
To investigate the photocatalytic performance of the composites under visible light, it was necessary to study the factors that influence photocatalytic activity. It is well known that the adsorption capacities of organic pollutants have an important influence on the photocatalytic performance. CR and MO were used in this study. CR and MO are anionic dyes with negatively charged groups in their molecular structure.21
The equilibrium uptake of these dyes can be calculated as follows:
 | (1) |
Fig. 7a and b show the changes in adsorption behavior with time for CR and MO, respectively, in the presence of CCTO, PANI, 90% PANI–10% CCTO, 70% PANI–30% CCTO and 50% PANI–50% CCTO. It can be seen that there was no significant adsorption of either dye on the surface of the CCTO particles, whereas there was significant adsorption on the surface of the PANI and PANI/CCTO nanofibers. This is a result of the chemical interaction between the electron-rich groups of the dyes and the positively charged backbone of PANI.20
 | ||
| Fig. 7 Changes in the adsorption capacity of (a) Congo red and (b) methyl orange with time in the presence of different catalysts. | ||
We therefore concluded that the adsorption of MO and CR on the PANI/CCTO nanofibers was slightly improved compared with PANI alone. In addition, the initial rate of adsorption of the PANI/CCTO composite was slightly higher than that of PANI alone. The equilibrium adsorption capacity, qe, also follows this order. This result shows that the adsorption capacity has some influence on the photocatalytic efficiency of the catalysts.43
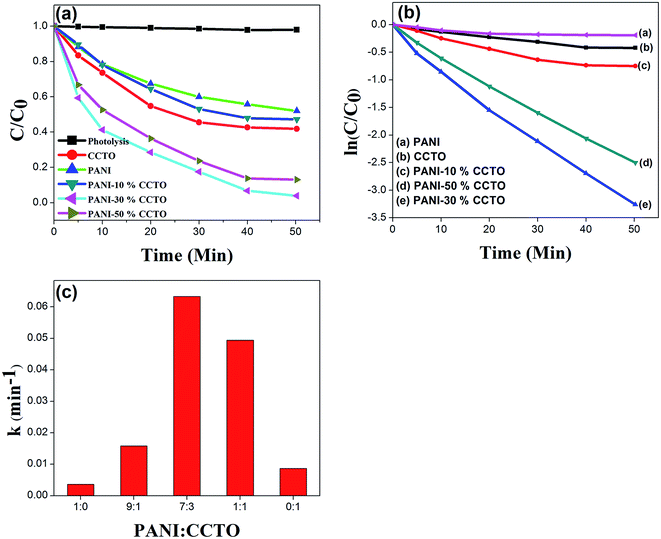
Photocatalytic degradation of the dyes using the synthesized catalysts was carried out under visible light irradiation. The photodegradation experiments were conducted after adsorption equilibrium had been obtained. The photodegradation rates of CR and MO on pure CCTO, pure PANI and in the CCTO–PANI nanofibers under visible light irradiation at 25 °C are shown in Fig. 8 and 9, respectively. It has been reported that polymers such as PANI with a semiconducting band gap show photocatalytic properties under UV light irradiation.44 PANI showed almost no activity under visible light irradiation with either CR or MO. It is interesting that CCTO is photocatalytically inactive under visible light, whereas the nanofiber composite of CCTO and PANI showed a dramatic enhancement in photocatalytic activity under visible light irradiation (Fig. 8a and 9a). The photodegradation process can be expressed by pseudo-first-order kinetics:
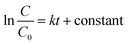 | (2) |
Fig. 8b and 9b show the kinetic decay plots for CR and MO, respectively. Fig. 8c and 9c show the observed degradation rate constants of CR and MO using photocatalysts with different weight ratios of PANI and CCTO. The photocatalytic degradation efficiency of the synthesized nanofiber composites were in the order: 30% CCTO + 70% PANI > 50% CCTO + 50% PANI > 10% CCTO + 90% PANI. Table 1 compares the degradation rate constants for selected PANI-based composites and synthesized PANI/CCTO nanofibers. Table 1 shows that the PANI/CCTO nanofibers had a better degradation efficiency for CR and MO than PANI-based composites reported previously.
A difference in degradation capability was observed in samples with different ratios of PANI/CCTO. The optimum photocatalytic performance was obtained for a ratio of PANI/CCTO of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, when the kinetic degradation constants for MO and CR were 0.056 and 0.064 min−1, respectively. An appropriate ratio of PANI results in uniform dispersion on the CCTO surface, which is beneficial to the transfer and separation of electrons and holes. At proportions of PANI above or below this value, the photodegradation efficiency was lower. When the molar ratio of PANI/CCTO was 1
3, when the kinetic degradation constants for MO and CR were 0.056 and 0.064 min−1, respectively. An appropriate ratio of PANI results in uniform dispersion on the CCTO surface, which is beneficial to the transfer and separation of electrons and holes. At proportions of PANI above or below this value, the photodegradation efficiency was lower. When the molar ratio of PANI/CCTO was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the percentage of PANI was too low to react with the CCTO nanoparticles completely. Therefore the number of adsorption and photocatalytic active sites was limited. However, as an electron donor, the PANI in the PANI/CCTO 1
1, the percentage of PANI was too low to react with the CCTO nanoparticles completely. Therefore the number of adsorption and photocatalytic active sites was limited. However, as an electron donor, the PANI in the PANI/CCTO 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 hybrid inevitably provides fewer electrons than other hybrids. Holes and electrons play important roles in the degradation of organic molecules because they are responsible for the production of hydroxyl radicals and superoxide anions. However, an excess of PANI in PANI/CCTO (9
1 hybrid inevitably provides fewer electrons than other hybrids. Holes and electrons play important roles in the degradation of organic molecules because they are responsible for the production of hydroxyl radicals and superoxide anions. However, an excess of PANI in PANI/CCTO (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) leads to a decrease in the degradation rate of MO and CR because the higher content of PANI nanoflakes results in overlapping agglomerates and obscures the hierarchical structure, hindering direct contact between CCTO and the dyes. All these factors result in a low photocatalytic activity. The composite with 30% CCTO + 70% PANI displayed a higher photodegradation efficiency than the other samples and therefore 30% CCTO + 70% PANI was selected for subsequent experiments.
1) leads to a decrease in the degradation rate of MO and CR because the higher content of PANI nanoflakes results in overlapping agglomerates and obscures the hierarchical structure, hindering direct contact between CCTO and the dyes. All these factors result in a low photocatalytic activity. The composite with 30% CCTO + 70% PANI displayed a higher photodegradation efficiency than the other samples and therefore 30% CCTO + 70% PANI was selected for subsequent experiments.
It has been reported that these dyes absorb visible light and become excited. The excited state can be degraded by self-sensitization when the energy level of the conduction band matches that of the photocatalysts. To demonstrate that the degradation of the dyes is realized through photocatalysis rather than through photosensitization, the CR and MO solutions were illuminated with visible light irradiation in the absence of photocatalysts. The results showed that, under such conditions, there was no apparent change in the dye concentration, indicating that the degradation of the dyes took place by photocatalysis alone rather than by photosensitization. As a result of the significant adsorption capacity of the CCTO–PANI nanofiber photocatalysts, we needed to confirm that the dyes were photodegraded rather than adsorbed onto the catalysts at the end of the photocatalytic reaction. Before irradiation, the photocatalytic systems were magnetically stirred for 2 h in the dark. The adsorption equilibrium of the dyes on the surface of the catalyst was reached, as proved by the adsorption kinetics experiment (Fig. 7). The dye concentration measured after equilibrium was used as the initial concentration and it is reasonable to conclude that the adsorption of the dye on the catalyst surface made little contribution to the variation in the concentration of the dye during the photocatalytic process.
The photocatalytic activity of the recycled PANI/CCTO nanofiber catalyst was also investigated and the results are shown in Fig. 10. There was no noticeable change in the photocatalytic activity of the recycled catalyst after four cycles under visible light irradiation, indicating that the nanofibrous PANI/CCTO was a stable and effective photocatalyst for the degradation of CR (Fig. 10a) and MO (Fig. 10b). A similar stability for PANI in other PANI-based photocatalysts has been found by several groups.20,30,32
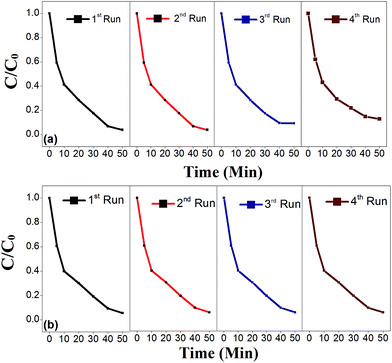 | ||
| Fig. 10 Cyclic photodegradation of (a) CR and (b) MO in the presence of a recyclable 70% PANI–30% CCTO photocatalyst under visible light irradiation. | ||
This significant enhancement in photoactivity can be attributed to the remarkable synergistic effect between CCTO and PANI. Transient photocurrent measurements were used to analyze the synergistic effect of the individual components. The transient photocurrent responses of pure CCTO, PANI and CCTO–PANI electrodes were recorded via several on–off cycles of irradiation. Fig. 11 shows the transient photocurrent responses under visible light and in the dark for samples where the photocurrents were measured at 0.0 V vs. SCE in 0.5 M aqueous Na2SO4. The responses of the photocurrent were reproducible during repeated on–off cycles under visible light irradiation. The higher photocurrent of the PANI/CCTO electrode results from the high mobility of the charge carriers generated by visible light irradiation.20 The photocurrent of the PANI/CCTO electrode (6 μA) was much higher than that of pure CCTO (2 μA) and pure PANI (0.6 μA), indicating that the separation efficiency of the photoinduced electrons and holes was enhanced through the electronic interaction between the CCTO nanoparticles and PANI.18
 | ||
| Fig. 11 Transient photocurrent responses of PANI, CCTO, and PANI/CCTO electrodes under visible light irradiation. | ||
To investigate the photocatalytic degradation mechanism, it is important to identify the main active oxidant in the photocatalytic reaction process. The active oxidants generated in the photocatalytic process can usually be measured by trapping with disodium EDTA-2Na (a hole scavenger) and t-BuOH (˙OH radical scavenger).45 As shown in Fig. 12a, in the PANI/CCTO photocatalyst system, the photodegradation of MO under visible light irradiation was obviously restrained after the injection of t-BuOH or EDTA-2Na, with both scavengers giving almost the same tendency to decrease with time. This phenomenon suggests that both radicals and holes are the main active species in this system.21 Fig. 12a and b show the visible light photocatalysis of MO and CR using PANI/CCTO nanofibers with hole and ˙OH radicle scavengers. It can be clearly seen that, after the addition of EDTA-2Na, there was little change in photodegradation in the PANI/CCTO suspension, whereas the addition of t-BuOH greatly reduced the photodegradation rate of MO, indicating that radicals were the main oxidant and that the dyes were eliminated mainly by oxygenous radical oxidation under visible light irradiation. This indicates that most of the holes could react with the adsorbed water (or hydroxide anions) to yield hydroxyl radicals and that only a few of them directly oxidized the MO molecules. Therefore we can conclude that OH radicals are the main oxidant in the PANI/CCTO system and that radical oxidation is the predominant reaction.
 | ||
| Fig. 12 Plots of photogenerated carriers trapped by PANI/CCTO in the photodegradation system of (a) CR and (b) MO under visible light irradiation. | ||
Fig. 13 shows a schematic mechanism for the photocatalytic reaction under visible light irradiation using the PANI/CCTO nanofiber photocatalyst. The MO and CR molecules have negatively charged groups that interact chemically with the positively charged backbone of PANI, leading to significant adsorption. Both CCTO and PANI can be excited by visible light to yield photogenerated carriers and excited electrons, respectively. The excited state electrons in PANI can readily migrate to the conduction band of CCTO. Simultaneously, the photogenerated holes in the valance band of CCTO can directly transfer to the HOMO of PANI.46 As PANI is a good material for transporting holes, the photogenerated charges can easily migrate to the surface of the photocatalysts and photodegrade the adsorbed species.
 | ||
| Fig. 13 Schematic diagram illustrating the synergistic effect of photocatalysis using a PANI/CCTO catalyst with visible light irradiation. | ||
Conclusions
A PANI/CCTO nanofibrous composite with a synergistic photodegradation effect was synthesized by controlled in situ polymerization. The photocatalytic degradation experiments showed that the as-prepared PANI/CCTO nanofiber catalyst had a high photocatalytic activity and adsorption capability toward anionic synthetic dyes (MO and CR) under natural sunlight under their individual optimum experimental conditions. The synthesized PANI/CCTO nanofibers had excellent stability and reusability in the photocatalytic degradation of MO and CR. The transient photocurrent experiments showed that PANI/CCTO had a synergistic effect on lower rates of recombination and efficient charge transport, which improves the photocatalytic performance of the catalyst. This study showed that the prepared nanofiber composite has good potential for the treatment of polluted waters.Acknowledgements
Rahul Vaish thanks the Indian National Science Academy, India for a research grant under INSA Young Scientists Scheme.Notes and references
- C. G. Daughton and T. A. Ternes, Environ. Health Perspect., 1999, 107, 907–938 CrossRef CAS.
- S. K. Khetan and T. J. Collins, Chem. Rev., 2007, 107, 2319–2364 CrossRef CAS PubMed.
- H. Ali, Water, Air, Soil Pollut., 2010, 213, 251–273 CrossRef CAS.
- A. B. dos Santos, F. J. Cervantes and J. B. van Lier, Bioresour. Technol., 2007, 98, 2369–2385 CrossRef CAS PubMed.
- F. Baquero, J.-L. Martínez and R. Cantón, Curr. Opin. Biotechnol., 2008, 19, 260–265 CrossRef CAS PubMed.
- J. Rivera-Utrilla, M. Sánchez-Polo, M. Á. Ferro-García, G. Prados-Joya and R. Ocampo-Pérez, Chemosphere, 2013, 93, 1268–1287 CrossRef CAS PubMed.
- N. Azbar, T. Yonar and K. Kestioglu, Chemosphere, 2004, 55, 35–43 CrossRef CAS PubMed.
- O. J. Hao, H. Kim and P.-C. Chiang, Crit. Rev. Environ. Sci. Technol., 2000, 30, 449–505 CrossRef CAS PubMed.
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- O. K. Dalrymple, D. H. Yeh and M. A. Trotz, J. Chem. Technol. Biotechnol., 2007, 82, 121–134 CrossRef CAS PubMed.
- A. Fujishima and K. Honda, Nature, 1972, 37–38 CrossRef CAS PubMed.
- N. Serpone and A. Emeline, J. Phys. Chem. Lett., 2012, 3, 673–677 CrossRef CAS PubMed.
- A. Mills, R. H. Davies and D. Worsley, Chem. Soc. Rev., 1993, 22, 417–425 RSC.
- K. Woan, G. Pyrgiotakis and W. Sigmund, Adv. Mater., 2009, 21, 2233–2239 CrossRef CAS PubMed.
- X. Lin, F. Rong, X. Ji, D. Fu and C. Yuan, Solid State Sci., 2011, 13, 1424–1428 CrossRef CAS PubMed.
- A. Sun, H. Chen, C. Song, F. Jiang, X. Wang and Y. Fu, RSC Adv., 2013, 3, 4332–4340 RSC.
- H. Fu, T. Xu, S. Zhu and Y. Zhu, Environ. Sci. Technol., 2008, 42, 8064–8069 CrossRef CAS.
- X. Li, D. Wang, G. Cheng, Q. Luo, J. An and Y. Wang, Appl. Catal., B, 2008, 81, 267–273 CrossRef CAS PubMed.
- K. Lee, S. Cho, S. H. Park, A. Heeger, C.-W. Lee and S.-H. Lee, Nature, 2006, 441, 65–68 CrossRef CAS PubMed.
- Y. Lin, D. Li, J. Hu, G. Xiao, J. Wang, W. Li and X. Fu, J. Phys. Chem. C, 2012, 116, 5764–5772 CAS.
- N. Guo, Y. Liang, S. Lan, L. Liu, J. Zhang, G. Ji and S. Gan, J. Phys. Chem. C, 2014, 118, 18343–18355 CAS.
- S. E. Shaheen, C. J. Brabec, N. S. Sariciftci, F. Padinger, T. Fromherz and J. C. Hummelen, Appl. Phys. Lett., 2001, 78, 841–843 CrossRef CAS PubMed.
- Y. Shirota and H. Kageyama, Chem. Rev., 2007, 107, 953–1010 CrossRef CAS PubMed.
- J. Shi and L. Guo, Prog. Nat. Sci., 2012, 22, 592–615 CrossRef PubMed.
- J. Ding, J. Bao, S. Sun, Z. Luo and C. Gao, J. Comb. Chem., 2009, 11, 523–526 CrossRef CAS PubMed.
- V. Ting, Y. Liu, L. Norén, R. Withers, D. Goossens, M. James and C. Ferraris, J. Solid State Chem., 2004, 177, 4428–4442 CrossRef CAS PubMed.
- J. H. Clark, M. S. Dyer, R. G. Palgrave, C. P. Ireland, J. R. Darwent, J. B. Claridge and M. J. Rosseinsky, J. Am. Chem. Soc., 2010, 133, 1016–1032 CrossRef PubMed.
- P. Thomas, K. Dwarakanath, K. Varma and T. Kutty, J. Therm. Anal. Calorim., 2009, 95, 267–272 CrossRef CAS.
- P. Thomas, K. Dwarakanath and K. Varma, J. Eur. Ceram. Soc., 2012, 32, 1681–1690 CrossRef CAS PubMed.
- P. Xiong, Q. Chen, M. He, X. Sun and X. Wang, J. Mater. Chem., 2012, 22, 17485–17493 RSC.
- H. Zhang, R. Zong, J. Zhao and Y. Zhu, Environ. Sci. Technol., 2008, 42, 3803–3807 CrossRef CAS.
- M. Shang, W. Wang, S. Sun, J. Ren, L. Zhou and L. Zhang, J. Phys. Chem. C, 2009, 113, 20228–20233 CAS.
- J. Hou, R. Cao, S. Jiao, H. Zhu and R. Kumar, Appl. Catal., B, 2011, 104, 399–406 CrossRef CAS PubMed.
- V. Eskizeybek, F. Sarı, H. Gülce, A. Gülce and A. Avcı, Appl. Catal., B, 2012, 119, 197–206 CrossRef PubMed.
- J. A. Khan, M. Qasim, B. R. Singh, S. Singh, M. Shoeb, W. Khan, D. Das and A. H. Naqvi, Spectrochim. Acta, Part A, 2013, 109, 313–321 CrossRef CAS PubMed.
- Y. Moon, Y. Cao, P. Smith and A. Heeger, Polym. Commun., 1989, 30, 196–199 CAS.
- S. Misra, M. Yadav and P. Mathur, Thin Solid Films, 2006, 514, 272–277 CrossRef CAS PubMed.
- W. Feng, E. Sun, A. Fujii, H. Wu, K. Niihara and K. Yoshino, Bull. Chem. Soc. Jpn., 2000, 73, 2627–2633 CrossRef CAS.
- J. Du, Z. Liu, B. Han, Z. Li, J. Zhang and Y. Huang, Microporous Mesoporous Mater., 2005, 84, 254–260 CrossRef CAS PubMed.
- M. V. Kulkarni, A. K. Viswanath, R. Marimuthu and T. Seth, Polym. Eng. Sci., 2004, 44, 1676–1681 CAS.
- P. Thomas, K. Dwarakanath and K. Varma, Synth. Met., 2009, 159, 2128–2134 CrossRef CAS PubMed.
- S. Palaniappan and B. Narayana, Thermochim. Acta, 1994, 237, 91–97 CrossRef CAS.
- T. Xu, Y. Cai and K. E. O'Shea, Environ. Sci. Technol., 2007, 41, 5471–5477 CrossRef CAS.
- B. Muktha, G. Madras, T. Guru Row, U. Scherf and S. Patil, J. Phys. Chem. B, 2007, 111, 7994–7998 CrossRef CAS PubMed.
- T. Xu, L. Zhang, H. Cheng and Y. Zhu, Appl. Catal., B, 2011, 101, 382–387 CrossRef CAS PubMed.
- J. Li, L. Zhu, Y. Wu, Y. Harima, A. Zhang and H. Tang, Polymer, 2006, 47, 7361–7367 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |