Self-delivery nanoparticles from an amphiphilic covalent drug couple of irinotecan and bendamustine for cancer combination chemotherapy†
Abstract
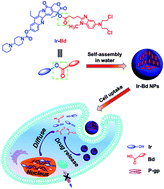
Over the past several decades, combination cancer chemotherapy has attracted scientists' extensive concern. Herein we constructed a drug self-delivery system with 100% drug content for combined cancer chemotherapy. In detail, the hydrophilic anticancer drug irinotecan (Ir) was directly coupled with the hydrophobic anticancer drug bendamustine (Bd) by esterification to form an amphiphilic covalent drug couple (Ir–Bd). Due to the amphiphilic property, Ir–Bd can self-assemble into nanoparticles (NPs) in water with an average diameter around 90.8 nm. Ir–Bd NPs show longer blood retention half-lifes, facilitate the accumulation of drugs in tumor tissues and promote cellular uptake comparing with free Bd or Ir. Benefiting from nanoscale characterizations, they can be efficiently uptaken by tumor cells to realize the self-delivery of both anticancer drugs without any carriers. Under the influence of the acidic environment within tumor cells, the ester bond between Ir and Bd is cleaved by hydrolyzation to release both free drugs of Ir and Bd simultaneously, which shows a marked synergistic action against tumor cells compared to that of single free Ir or Bd because of the non-overlapping toxicity profile of Ir and Bd. In addition, the multidrug resistance (MDR) of tumor cells can also be avoided efficiently by profiting from the nanoscale characteristic of Ir–Bd NPs.

 Please wait while we load your content...
Please wait while we load your content...