One-step fabrication of polymeric hybrid particles with core–shell, patchy, patchy Janus and Janus architectures via a microfluidic-assisted phase separation process†
Xiaodong Caoabc,
Wenxiu Liabc,
Ting Maab and
Hua Dong *abc
*abc
aDepartment of Biomedical Engineering, School of Materials Science and Engineering, South China University of Technology, Guangzhou, 510006, China. E-mail: donghua@scut.edu.cn
bNational Engineering Research Center for Tissue Restoration and Reconstruction, Guangzhou, 510641, China
cGuangdong Province Key Laboratory of Biomedical Engineering, South China University of Technology, Guangzhou, 510641, China
First published on 15th September 2015
Abstract
We report in this paper a one-step route for the preparation of core–shell, patchy, patchy Janus and Janus particles via a microfluidic-assisted phase separation process. As a proof of concept, PLGA/PCL hybrid particles are fabricated and the results show that the four types of particles can be harvested with high yield and narrow size distribution by precise control over the phase separation process, or namely, interfacial tensions and spreading coefficients between immiscible phases, after generation of the single emulsion in microchannels. This new strategy described herein is rather simple and versatile for producing a wide range of other particles with simultaneously controlled size, shape and anisotropy.
Introduction
Anisotropic particles with patchy, multi-compartmental and Janus architectures have attracted considerable interest from the materials science community, considering not only their unique physical/chemical properties and functions, but also their promising applications in photonics, solid surfactants, targeted drug delivery, biomimetic colloidal building blocks, biomedical imaging and biosensors.1–8 In the past few years, numerous innovative strategies have been developed to design and prepare anisotropic particles, including self-assembly, Pickering emulsions, particle lithography, surface modification, photo-polymerization, biphasic electro-jetting/spraying etc.9–19 For example, Du and Chen reported a patchy inorganic–organic hybrid vesicle via self-assembly of a reactive amphiphilic block copolymer, followed by a stabilization step using a sol–gel process.20 Lahann's group described a novel route for producing multi-compartmental particles using electrohydrodynamic co-jetting of composite solution.21 Liu et al. demonstrated the formation of amphiphilic Janus particles through a two-phase redox-initiated polymerization of silica particles stabilizing a water-in-oil Pickering emulsion.22 Despite of the great success in fabricating separate type of anisotropic particles, these methods show poor flexibility to switch among distinct particle configurations without altering the particle chemistry, severely limiting their practical applications. Hence, there still remains a great demand to explore facile yet versatile approaches for harvesting manifold anisotropic particles via similar protocols and parameters.In our earlier paper,23 we presented a simple method to synthesize Janus and microcapsule particles via droplet-based microfluidic technology. Herein, we further show one-step fabrication of core–shell, patchy, patchy Janus and Janus particles by subtle combination of droplet-based microfluidics and phase separation (Scheme 1). With the assistance of droplet-based microfluidics, oil-in-water (O/W) single emulsions with highly uniform size can be generated (Fig. S1, ESI†), which promises the homogeneity in the subsequent phase separation process and thus the high yield in one specific particle type. In our opinion, the key point to realize the diversity in particle architectures lies in the precise control over the phase separation of a droplet under various conditions, or namely, interfacial tensions and spreading coefficients between immiscible phases. In particular, we demonstrate that interfacial tensions and spreading coefficients can be deliberately and continuously tuned in our system, resulting in variable but controllable phase separation from single emulsion and then distinct particle anisotropy.
Experimental section
Materials
Poly(lactic-co-glycolic acid) (PLGA, lactide/glycolide ratio = 50/50, Mw = 30 kDa) and poly(ε-caprolactone) (PCL, Mw = 130 kDa) were purchased from Daigang Biomaterials (Shandong, China). Poly(vinyl alcohol) (PVA, 87–89% hydrolyzed, Mw = 88 kDa), rhodamine B and dimethyl carbonate were bought from Aladdin Chemistry (Shanghai, China). Dichloromethane and acetone were obtained from Tianjing Baishi Chemical Co. Ltd (Tianjin, China). Glycerol was purchased from Tianjin Fuyu Chemical Co. Ltd (Tianjin, China). Polydimethylsiloxane (PDMS) (Sylgard 184) and negative resist (NR21-20000P) were bought from Dow Corning Company and Futurrex Inc (USA), respectively. Deionized water was obtained using a Milli-Q water-purification system. All the reagents were of analytical grade and used without further purification.Fabrication and surface modification of microfluidic devices
Droplet-based microfluidic devices with flow-focusing structure were fabricated via standard soft lithography techniques, as reported in our earlier work.24 In brief, clean silicon wafer was first spin-coated with negative photoresist. After baking at 80 °C for 10 min and 150 °C for 5 min, the resist was exposed to UV light through a photo-mask and developed in RD6 developer solution. Mixture of PDMS base and curing agent (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) was then poured onto the silicon wafer, degassed by vacuum oven and cured on a hotplate at 60 °C. The PDMS replica was subsequently peeled off and sealed with a glass slide via O2 plasma. The width of branch channel and collection channel were 100 μm and 250 μm, and the depth of all channels was 100 μm (see schematic illustration of the device in Fig. S1a†). To improve the hydrophilicity of microchannels and reduce the swelling problem of PDMS caused by dichloromethane, surface modification was conducted by injecting PVA/glycerol (2/5 wt%) aqueous solution and curing for 1 h.
1, w/w) was then poured onto the silicon wafer, degassed by vacuum oven and cured on a hotplate at 60 °C. The PDMS replica was subsequently peeled off and sealed with a glass slide via O2 plasma. The width of branch channel and collection channel were 100 μm and 250 μm, and the depth of all channels was 100 μm (see schematic illustration of the device in Fig. S1a†). To improve the hydrophilicity of microchannels and reduce the swelling problem of PDMS caused by dichloromethane, surface modification was conducted by injecting PVA/glycerol (2/5 wt%) aqueous solution and curing for 1 h.
Synthesis of anisotropic PLGA/PCL hybrid particles
PLGA and PCL (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5
7, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or 7
5 or 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/w) dissolved in dichloromethane, dimethyl carbonate or their mixture (5
3, w/w) dissolved in dichloromethane, dimethyl carbonate or their mixture (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) with a total concentration of 40 mg mL−1 were used as the dispersed phase, whilst an aqueous solution containing 2 wt% of PVA was used as the continuous phase. Both the dispersed phase and continuous phase were delivered into the microfluidic channels using syringe pumps (Cole-Parmer, USA). Before injecting the dispersed phase, the microchannels were wetted with the continuous phase for several minutes to benefit the formation of O/W droplets. For microcapsule and patchy particles, the flow rates of the continuous and dispersed phases were set as 0.25 and 0.45 mL h−1 (the corresponding yield was ∼2.7 × 105 h−1). In the case of Janus and patchy Janus particles, the flow rates for the continuous and dispersed phases were held at 0.4 and 1.6 mL h−1 (the corresponding yield was ∼1.2 × 106 h−1). Droplets were generated continuously at the junction of the microchannels and then collected in 2 wt% aqueous solution of PVA (Fig. S1b and c†). After settling at room temperature for 24 h, the solidified microparticles were centrifuged, washed for 3 times with DI water and finally dried in a freeze-drier (Lyophilizer, VIRTIS, USA).
2, v/v) with a total concentration of 40 mg mL−1 were used as the dispersed phase, whilst an aqueous solution containing 2 wt% of PVA was used as the continuous phase. Both the dispersed phase and continuous phase were delivered into the microfluidic channels using syringe pumps (Cole-Parmer, USA). Before injecting the dispersed phase, the microchannels were wetted with the continuous phase for several minutes to benefit the formation of O/W droplets. For microcapsule and patchy particles, the flow rates of the continuous and dispersed phases were set as 0.25 and 0.45 mL h−1 (the corresponding yield was ∼2.7 × 105 h−1). In the case of Janus and patchy Janus particles, the flow rates for the continuous and dispersed phases were held at 0.4 and 1.6 mL h−1 (the corresponding yield was ∼1.2 × 106 h−1). Droplets were generated continuously at the junction of the microchannels and then collected in 2 wt% aqueous solution of PVA (Fig. S1b and c†). After settling at room temperature for 24 h, the solidified microparticles were centrifuged, washed for 3 times with DI water and finally dried in a freeze-drier (Lyophilizer, VIRTIS, USA).
Characterization of anisotropic PLGA/PCL particles
The particle fabrication process were monitored using an optical microscope (T240C, SunTime, China) equipped with a high-speed camera (Basler ace, Germany). Rhodamine B-labeled particles were observed with a confocal laser scanning microscope (CLSM) equipped with a 1 mW helium–neon laser (ZeissLsm-510, Japan). The particle morphology was detected via a scanning microscope (Nova Nano SEM430, FEI). Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR, Nexus Por Euro, USA) and X-ray diffraction (D8 ADVANCE, Bruker, Germany) were carried out to further clarify the particle configuration. The interfacial tension was measured at 25 °C with the Wilhelmy plate method (DCAT 30, Dataphysics instruments GmbH), and the polymer surface energy was determined according to the classic Owens–Wendt–Kaelble (OWK) method.Results and discussion
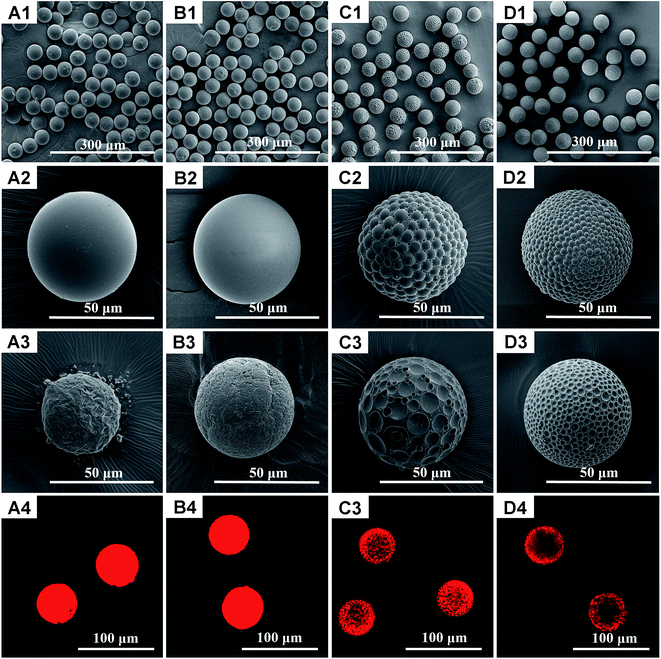
Phase separation in single emulsion is essentially determined by interfacial tensions and spreading coefficients between immiscible phases, which are affected by many factors. It is found in our study that interfacial tensions and spreading coefficients can be deliberately and precisely tuned via the volume ratio of mixed solvents in the dispersed phase and mass ratio of polymer components. In another word, particles with significantly different architectures can be fabricated simply by fine tuning of experimental parameters. As a proof of concept, polymeric hybrid particles composed of immiscible poly (lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL), due to their excellent biocompatibility and biodegradability as well as their complementary properties in mechanical strength, degradation rate and hydrophobility/hydrophilicity etc23, were chosen as model materials. Fig. 1 shows the scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) images of PLGA/PCL particles prepared using pure dichloromethane as the organic solvent for the dispersed phase. Either core–shell or patchy particle shapes can be observed, depending on the mass ratio between PLGA and PCL. More specifically, when the mass ratios of PLGA/PCL are 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 5
3 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, core–shell particles are harvested with high productivity. Instead, when the PLGA/PCL mass ratios change to 3
5, core–shell particles are harvested with high productivity. Instead, when the PLGA/PCL mass ratios change to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 2
7 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, anisotropic patchy particles can be both obtained, expect that surface patches are bigger at higher mass ratio (3
8, anisotropic patchy particles can be both obtained, expect that surface patches are bigger at higher mass ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). To figure out the distribution of PLGA and PCL on the particle surface, acetone treatment was introduced to selectively dissolve PLGA, because acetone is a good solvent for PLGA but relatively difficult to dissolve PCL in a very short time.25 As can be seen, core–shell particles still maintain an approximately spherical structure after treated with acetone but the particle surface is not smooth anymore, which proves the original shell is made of PLGA. The rough surface of remaining particle might be attributed to the semi-crystalline state of PCL. The smaller particle size in Fig. 1(A3) implies a thicker PLGA shell in the untreated core–shell particles prepared under the mass ratio of 7
7). To figure out the distribution of PLGA and PCL on the particle surface, acetone treatment was introduced to selectively dissolve PLGA, because acetone is a good solvent for PLGA but relatively difficult to dissolve PCL in a very short time.25 As can be seen, core–shell particles still maintain an approximately spherical structure after treated with acetone but the particle surface is not smooth anymore, which proves the original shell is made of PLGA. The rough surface of remaining particle might be attributed to the semi-crystalline state of PCL. The smaller particle size in Fig. 1(A3) implies a thicker PLGA shell in the untreated core–shell particles prepared under the mass ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. On the other hand, patchy particles after acetone treatment show many surface dimples and the whole structure turns out to be golf ball-like, confirming that surface patches are composed of PLGA. Surprisingly, although core–shell and patchy particles exhibit distinct surface morphologies, their inner structures are quite similar, as supported by the SEM images of cross-sectioned particles where many tiny PLGA beads are encapsulated in the PCL matrix (Fig. S2, ESI†). In addition, rhodamine B, a hydrophilic fluorescent dye, was also used to stain hydrophilic PLGA in the PLGA/PCL particles with the CLSM images shown in Fig. 1(A4–D4). The locations of PLGA highlighted by rhodamine B are in good agreement with the conclusions drawn from acetone treatment.
3. On the other hand, patchy particles after acetone treatment show many surface dimples and the whole structure turns out to be golf ball-like, confirming that surface patches are composed of PLGA. Surprisingly, although core–shell and patchy particles exhibit distinct surface morphologies, their inner structures are quite similar, as supported by the SEM images of cross-sectioned particles where many tiny PLGA beads are encapsulated in the PCL matrix (Fig. S2, ESI†). In addition, rhodamine B, a hydrophilic fluorescent dye, was also used to stain hydrophilic PLGA in the PLGA/PCL particles with the CLSM images shown in Fig. 1(A4–D4). The locations of PLGA highlighted by rhodamine B are in good agreement with the conclusions drawn from acetone treatment.
In contrast, if dimethyl carbonate was employed as the organic solvent, the as-prepared particles show a typical Janus architecture with two well-defined regions and an apparent demarcation in between, indicative of the complete phase separation in the formation of such particles (Fig. 2). The shift of the demarcation line towards either the smooth or rough compartments with the variation in PLGA/PCL mass ratios verifies our anticipation, i.e., the smooth compartment belongs to PLGA whilst the rough one belongs to PCL. Once subjected to acetone for 30 s, all the Janus particles lose their PLGA components and the residual PCL becomes chesspiece-like, hemispheric and pileus-like respectively, as shown in Fig. 2(A3–C3). Interestingly, rhodamine B-stained Janus particles delineate the shape of PLGA compartments that are dissolved away in acetone treatment. Considering the significant difference in particle morphologies achieved by sole dichloromethane or dimethyl carbonate, it is conceivable of a transition structure in the case of mixed solvents. Fig. 3 presents the particles fabricated under two volume ratios of dichloromethane and dimethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and 2
5 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and a constant mass ratio of PLGA/PCL (5
1) and a constant mass ratio of PLGA/PCL (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). It is clear that patchy Janus particles with a smooth hemisphere and a patchy hemisphere are finally formed. Compared with the particles shown in Fig. 3(A2), the demarcation line of the particle in Fig. 3(B2), intrudes towards the smooth hemisphere when the volume ratio of solvents is 2
5). It is clear that patchy Janus particles with a smooth hemisphere and a patchy hemisphere are finally formed. Compared with the particles shown in Fig. 3(A2), the demarcation line of the particle in Fig. 3(B2), intrudes towards the smooth hemisphere when the volume ratio of solvents is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The acetone treatment reveals that the smooth hemisphere and surface patches on the other hemisphere disappear, suggesting that the missing parts are PLGA and the matrix of the patchy hemisphere is PCL. Similarly, CLSM images visualize the PLGA components on the particle surface and further prove patchy Janus structure.
1. The acetone treatment reveals that the smooth hemisphere and surface patches on the other hemisphere disappear, suggesting that the missing parts are PLGA and the matrix of the patchy hemisphere is PCL. Similarly, CLSM images visualize the PLGA components on the particle surface and further prove patchy Janus structure.
In addition to acetone treatment and CLSM measurement, attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and X-ray diffraction (XRD) were also recorded to identify the distribution of PLGA and PCL in the above-mentioned particles. The FTIR spectra in Fig. 4A show that only the characteristic peaks of PLGA at 2998, 2951, 1759, 1129 and 1086 cm−1 can be detected on the core–shell particle surface. This phenomenon implies that PCL is embedded inside the core–shell particles. Comparatively, except the PLGA peaks at 2997 and 1755 cm−1, the PCL peaks at 2946, 2865, 1737 and 731 cm−1 are also observed for patchy and Janus particles, which confirms the co-existence of PLGA and PCL on these two particle surfaces. Meanwhile, the XRD profiles of core–shell, patchy and Janus particles in Fig. 4B all display the diffraction peaks of amorphous PLGA and crystalline PCL, but the diffraction peak of PLGA is more remarkable on core–shell particles. A possible explanation is that only PLGA phase occupies the outside shell of core–shell particles.
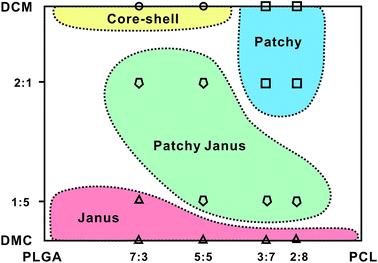
Fig. 5 summarizes the effects of the volume ratio of dichloromethane/dimethyl carbonate and mass ratio of PLGA/PCL on particle structures. High volume ratio of dichloromethane/dimethyl carbonate and high mass ratio of PLGA/PCL benefit the fabrication of core–shell particles, whereas high volume ratio of dichloromethane/dimethyl carbonate and low mass ratio of PLGA/PCL yields predominantly patchy particles. In comparison, Janus particles show weak relationship with the mass ratio of PLGA/PCL and can be generated under low volume ratio of dichloromethane/dimethyl carbonate. If medium volume ratio of dichloromethane/dimethyl carbonate and medium mass ratio of PLGA/PCL are used, anisotropic patchy Janus particles are the main products.
Although monodisperse core–shell, patchy, patchy Janus and Janus particles can be fabricated using the same device and analogous conditions, it should be pointed out that the particle type and size is almost invariable in terms of given parameters (see average particle size and size distribution in Fig. S3 and Table S1, ESI†), mainly owing to precise control over droplet formation via microfluidic devices and phase separation during solvent extraction.26 As we know, the equilibrium type of two immiscible phases in a third phase can be theoretically predicted from the interactive interfacial tensions between different phases (γij, γik, γjk) and spreading coefficients (Si),27 as stated in eqn (1):
| Si = γjk − (γij + γik) | (1) |
In our system, the oil phase containing PLGA, the aqueous solution of 2 wt% PVA and the oil phase containing PCL are defined as phase 1, 2 and 3, respectively. Table 1 lists the interfacial tensions measured via the Wilhelmy plate method and the spreading coefficients calculated using eqn (1). Since the solubility of dichloromethane in water is quite low (2 wt% at 20 °C), the dissolved PLGA and PCL in dichloromethane or its mixture with dimethyl carbonate are completely miscible for a long time (∼3–4 h for pure dichloromethane). Therefore, their interfacial tension γ13 is assumed as zero. Compared with dichloromethane, dimethyl carbonate shows much higher solubility in water (13.9 wt% at 20 °C20) and thus can be extracted from the emulsion quickly (<30 s). As a result, the interfacial tension between PLGA and PCL dissolved in dimethyl carbonate cannot be measured via the Wilhelmy plate method and is actually calculated based on their surface energy (see Table S2 for detailed measurement and calculation, ESI†).
| DCM/DMC (v/v) | PLGA/PCL (w/w) | γ12 (mN m−1) | γ13 (mN m−1) | γ23 (mN m−1) | S1 (mN m−1) | S2 (mN m−1) | S3 (mN m−1) | Equilibrium type | Particle structure |
|---|---|---|---|---|---|---|---|---|---|
| a Note: γ12 and γ23 were measured via the Wilhelmy plate method. γ13 was assumed as 0 for PLGA–PVA–PCL systems containing dichloromethane (DCM) and calculated via the surface energy of PLGA and PCL for PLGA–PVA–PCL systems containing pure dimethyl carbonate (DMC). The total concentrations of PLGA and PCL in the oil phase were 40 mg mL−1 except the sample marked by * where the total concentration was 80 mg mL−1. | |||||||||
| DCM | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.620 | 0 | 1.016 | +0.396 | −1.636 | −0.395 | Wetting | Core–shell |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
2.170 | 0 | 2.330 | +0.160 | −4.500 | −0.160 | Wetting | Core–shell | |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5* 5* |
2.87* | 0* | 1.72* | −1.15* | −4.59* | +1.15* | Wetting* | Core–shell* | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
1.381 | 0 | 0.933 | −0.448 | −2.314 | +0.448 | Wetting | Patchy | |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
1.367 | 0 | 1.197 | −0.170 | −2.564 | +0.170 | Wetting | Patchy | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.005 | 0 | 0.765 | −0.240 | −1.770 | +0.240 | Wetting | Patchy Janus |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.151 | 0 | −0.022 | −1.173 | −1.129 | +1.173 | Wetting | Patchy Janus |
| DMC | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
2.946 | 7.480 | 2.581 | −7.845 | +1.953 | −7.115 | Dewetting | Janus |
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3.080 | 7.480 | 2.920 | −7.640 | +1.480 | −7.320 | Dewetting | Janus | |
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
3.585 | 7.480 | 3.123 | −7.942 | +0.772 | −7.018 | Dewetting | Janus | |
With respect to the emulsion using dichloromethane as organic solvent, the signs of S1, S2 and S3 are variable, as functions of the mass ratio of PLGA/PCL and total concentration of these two components. At high PLGA/PCL mass ratio (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 5
3 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and low total concentration (for example, the initial total concentration of 40 mg mL−1), the positive S1, negative S2 and S3 meet the criteria of complete wetting, indicative of a core–shell structure with PLGA as the shell and PCL as the core. With the extraction of more dichloromethane into the water, the total concentration of PLGA and PCL increases, accompanying by the increase of γ12 and decrease of γ23 (in our study, 80 mg mL−1 was used for the measurement of γ12 and γ23 at high total concentration). This triggers the inversion of S1 and S3, leading to the encapsulation of PLGA in the PCL matrix inside the particle. However, the movement of PLGA molecules would be greatly slowed down in high-molecular-weight PCL matrix and thus the coalescence of tiny PLGA beads into larger PLGA core are restricted. At low PLGA/PCL mass ratio (3
5) and low total concentration (for example, the initial total concentration of 40 mg mL−1), the positive S1, negative S2 and S3 meet the criteria of complete wetting, indicative of a core–shell structure with PLGA as the shell and PCL as the core. With the extraction of more dichloromethane into the water, the total concentration of PLGA and PCL increases, accompanying by the increase of γ12 and decrease of γ23 (in our study, 80 mg mL−1 was used for the measurement of γ12 and γ23 at high total concentration). This triggers the inversion of S1 and S3, leading to the encapsulation of PLGA in the PCL matrix inside the particle. However, the movement of PLGA molecules would be greatly slowed down in high-molecular-weight PCL matrix and thus the coalescence of tiny PLGA beads into larger PLGA core are restricted. At low PLGA/PCL mass ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 2
7 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), only S3 is positive regardless of the total concentration. In principle, PLGA should be encapsulated into the PCL matrix during the solidification process. However, the PLGA beads formed at the particle surface by phase separation show low mobility across the PCL matrix, which causes the formation of surface patches.
8), only S3 is positive regardless of the total concentration. In principle, PLGA should be encapsulated into the PCL matrix during the solidification process. However, the PLGA beads formed at the particle surface by phase separation show low mobility across the PCL matrix, which causes the formation of surface patches.
Instead of the complexity induced by dichloromethane, the equilibrium type of emulsion using dimethyl carbonate is quite stable, i.e., the spreading coefficient S2 is always positive and the other two (S1 and S3) are negative, satisfying the condition of dewetting. As a consequence, only Janus particles can be fabricated, no matter how to change the mass ratio of PLGA/PCL. In the case of the mixed solvents, the measurements show S1 < 0, S2 < 0, and S3 > 0, similar to the results of patchy particles. However, due to the presence of dimethyl carbonate and its high solubility in water, phase separation between PLGA and PCL in mixed solvents starts dynamically according to dewetting mode just like pure dimethyl carbonate before it completely diffuses into water. Thereafter, phase separation continues according to wetting mode like pure dichloromethane. The former causes the formation of Janus structure whereas the latter results in patchy structure, which finally combines into patchy Janus shape. Obviously, the volume percentage of patchy compartment in patchy Janus particles can be tuned via the volume ratio of dichloromethane and dimethyl carbonate. As the volume ratio of dichloromethane and dimethyl carbonate increases (for example, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), it takes more time to finish the first-step of phase separation (dewetting mode) and thus the patchy compartment is smaller.
5), it takes more time to finish the first-step of phase separation (dewetting mode) and thus the patchy compartment is smaller.
Nevertheless, although only PLGA/PCL particles were investigated in the current study, our new strategy can be adopted to fabricate a wide range of other particles, if the corresponding emulsion system can meet the requirements predicted by the spreading and partial wetting theory, i.e., S1 > 0, S2 < 0 and S3 < 0 for core–shell structure, S1 < 0, S2 < 0 and S3 > 0 for patchy or patchy Janus structure, S1 < 0, S2 > 0 and S3 < 0 for Janus structure.
Conclusions
In summary, the beauty of this work is to develop a facile method for the fabrication of core–shell, patchy, patchy Janus and Janus particles under the same strategy (microfluidic-assisted phase separation) and similar conditions/parameters. Using PLGA/PCL as model materials, it has been vividly shown that the four types of particles can be produced with high yield and narrow size distribution by precisely controlling the phase separation process, or namely, interfacial tensions and spreading coefficients between immiscible phases, after generation of the single emulsion in microchannels. The inherent formation mechanisms of these particles are investigated and discussed in terms of the spreading and partial wetting theory. This new principle and strategy demonstrated herein is rather simple and versatile for producing a wide range of other particles with simultaneously controlled size, shape and anisotropy, and more encouragingly, no complex set-up and environment are required. Furthermore, in view of the vast difference of PLGA and PCL in mechanical strength, hydrophobility/hydrophilicity and biodegradation rate, core–shell, patchy, patchy Janus and Janus structured PLGA/PCL particles hold great promises in applications of controllable drug delivery, cell targeting and biomedical imaging.Acknowledgements
This work was financially sponsored by the National Natural Science Foundation of China (Grant No. 51373056, 51372085), Guangdong-Hongkong common technology bidding project (No. 2013B010136003) and Science and Technology Program of Guangdong Province of China (No. 2012A061100002).References
- S. Jiang, Q. Chen, M. Tripathy, E. Luijten, K. S. Schweizer and S. Granick, Adv. Mater., 2010, 22, 1060 CrossRef CAS PubMed.
- D. Dendukuri and P. S. Doyle, Adv. Mater., 2009, 21, 4071 CrossRef CAS PubMed.
- C. Tang, C. L. Zhang, Y. J. Sun, F. X. Liang, Q. Wang, J. L. Li, X. Z. Qu and Z. Z. Yang, Macromolecules, 2013, 46, 188 CrossRef CAS.
- A. Walther and A. H. E. Müller, Chem. Rev., 2013, 113, 5194 CrossRef CAS PubMed.
- V. N. Manoharan, M. T. Elsesser and D. J. Pine, Science, 2003, 301, 483 CrossRef CAS PubMed.
- J. Z. Du and R. K. O'Reilly, Chem. Soc. Rev., 2011, 40, 2402 RSC.
- L. Hong, S. Jiang and S. Granick, Langmuir, 2006, 22, 9495 CrossRef CAS PubMed.
- H. Unsal and N. Aydogan, Langmuir, 2009, 25, 7884 CrossRef CAS PubMed.
- H. Y. Koo, D. K. Yi, S. J. Yoo and D. Y. Kim, Adv. Mater., 2004, 16, 274 CrossRef CAS PubMed.
- K. H. Roh, D. C. Martin and J. Lahann, Nat. Mater., 2005, 4, 759 CrossRef CAS PubMed.
- K. D. Anderson, M. Luo, R. Jakubiak, R. R. Naik, T. J. Bunning and V. V. Tsukruk, Chem. Mater., 2010, 22, 3259 CrossRef CAS.
- Y. H. Chen, G. Nurumbetov, R. Chen, N. Ballard and S. A. F. Bon, Langmuir, 2013, 29, 12657 CrossRef CAS PubMed.
- J. W. J. de Folter, E. M. Hutter, S. I. R. Castillo, K. E. Klop, A. P. Philipse and W. K. Kegel, Langmuir, 2014, 30, 955 CrossRef CAS PubMed.
- D. J. Kraft, J. Hilhorst, M. A. P. Heinen, M. J. Hoogenraad, B. Luigjes and W. K. Kegel, J. Phys. Chem. B, 2013, 117, 2827 CrossRef CAS.
- B. G. P. van Ravensteijn, M. Kamp, A. van Blaaderen and W. K. Kegel, Chem. Mater., 2013, 25, 4348 CrossRef CAS.
- B. T. T. Pham, C. H. Such and B. S. Hawkett, Polym. Chem., 2015, 6, 426 RSC.
- Y. Zhang, H. R. Liu and F. W. Wang, Colloid Polym. Sci., 2013, 291, 2993 CAS.
- J. Song, H. Liu, M. X. Wan, Y. Zhu and L. Jiang, J. Mater. Chem. A, 2013, 1, 1740 CAS.
- Y. Nakagawa, H. Kageyama, Y. Oaki and H. Imai, J. Am. Chem. Soc., 2014, 136, 3716 CrossRef CAS PubMed.
- J. Du and Y. Chen, Angew. Chem., Int. Ed., 2004, 43, 5084 CrossRef CAS PubMed.
- W. P. Lv, K. J. Lee, J. J. Li, T. H. Park, S. Hwang, A. J. Hart, F. B. Zhang and J. Lahann, Small, 2012, 8, 3116 CrossRef CAS PubMed.
- B. Liu, W. Wei, X. Z. Qu and Z. Z. Yang, Angew. Chem., Int. Ed., 2008, 47, 3973 CrossRef CAS PubMed.
- W. X. Li, H. Dong, G. N. Tang, T. Ma and X. D. Cao, RSC Adv., 2015, 5, 23181 RSC.
- G. N. Tang, W. X. Li, X. D. Cao and H. Dong, RSC Adv., 2015, 5, 12872 RSC.
- P. N. Nge, C. I. Rogers and A. T. Woolley, Chem. Rev., 2013, 113, 2550 CrossRef CAS PubMed.
- X. P. Huang, Q. P. Qian and Y. P. Wang, Small, 2014, 10, 1412 CrossRef CAS PubMed.
- P. Tundo and M. Selva, Acc. Chem. Res., 2002, 35, 706 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16504k |
| This journal is © The Royal Society of Chemistry 2015 |