Highly porous N-doped TiO2 hollow fibers with internal three-dimensional interconnected nanotubes for photocatalytic hydrogen production
Kuang-I Liu,
Chung-Yi Su and
Tsong-Pyng Perng*
Department of Materials Science and Engineering, National Tsing Hua University, Hsinchu 300, Taiwan. E-mail: tpperng@mx.nthu.du.tw; Fax: +886-3-5723857; Tel: +886-3-5742634
First published on 12th October 2015
Abstract
Fabrication of TiO2 hollow fibers was conducted by atomic layer deposition (ALD) with polysulfone fibers (PSFs) as a template. After the ALD process, the PSFs were removed by heat treatment at 500 °C for 1 h to form anatase TiO2 hollow fibers. They were then doped with nitrogen up to ∼5 at% by treatment in NH3 at 500–600 °C. The N-doped TiO2 hollow fibers remained porous and the wall was full of three-dimensional interconnected nanotubes. The X-ray photoelectron spectroscopic analysis indicated that nitrogen was substitutionally doped into oxygen sites of the TiO2 lattice, resulting in band gap narrowing and more absorption in the visible light region. They exhibited significantly improved photocatalytic activity for water splitting due to a lower energy gap, higher reactive surface area (∼100 m2 g−1), multiple light reflection, and better charge separation efficiency. The fibers treated at 600 °C for 1 h, containing ∼2.5 at% nitrogen, generated 0.185 μmol g−1 of hydrogen after 6 h of irradiation with a 150 W Xe lamp.
Introduction
Photoelectrochemical splitting of water by TiO2 was first demonstrated in the early 1970s by Fujishima and Honda.1 Titanium dioxide, as an inexpensive, nontoxic, chemically stable, and highly efficient photocatalyst, has been extensively applied for degradation of organic pollutants, air purification, and photocatalytic hydrogen evolution.2,3 However, due to its wide band gap (3.2 eV), only about 4% of the solar energy on the earth's surface can be utilized. Therefore, it is important to develop novel anatase-based materials with an appropriate band gap to fit the visible spectral region. Impurity doping is one of the typical approaches to extend the spectral response of titanium dioxide to visible light. Some metal dopants such as Cr, Co, W, Mo, and V have been shown to enhance the photocatalytic activity of TiO2,4,5 nevertheless, metal doping may have disadvantages of thermal instability and carrier trapping. It is well known that the preparation of transition metal-doped TiO2 by ion implantation technique is more expensive.6 Recently, it has been observed that TiO2 doped with nonmetals, such as F, S, and N, would efficiently extend photoresponse from UV light region to visible light region.7–9 N-doped TiO2 was first reported to exhibit good visible light photocatalytic activity by Sato.10 Nitrogen doping seems to have caught more attention than other anionic elements due to small ionization energy and stability. The preparation method and condition greatly affect nitrogen doping. N-doped TiO2 has been fabricated by various methods such as ion implantation,11 pulsed laser deposition,12 chemical vapor deposition,13 and sputtering deposition.14 In addition, nitrogen doping can also be obtained by heating TiO2 in an ammonia atmosphere.Inorganic hollow fibers have attracted considerable attention because of versatile potential applications. Several techniques have been employed to fabricate inorganic hollow fibers such as electrophoretic deposition,15 template,16 and electrospinning.17 Among these methods, the template method is a simple and easy way to control the dimensions of hollow fibers. Polysulfone is a popular membrane polymer, which can be used as a template to fabricate nanomaterials because of its thermal, mechanical, and chemical stability.18 Thin films of TiO2 on nanostructured template have been produced by several techniques. The technique of ALD has advantages of conformal coating, precise thickness control, and good uniformity on large area for fabricating nanostructures.19 ALD is based on the sequential saturated surface reactions, where each precursor is alternately pulsed to the reaction chamber, and the reactions between the precursors and surface species are self-terminating. As a result, atomic level control of film growth can be achieved. Nowadays it has become a promising deposition method for fabricating thin film materials. In this study, we have developed a facile method to deposit TiO2 by ALD on polysulfone hollow fiber template to form TiO2 hollow fibers. Furthermore, N-doping was conducted by annealing the TiO2 coated polysulfone hollow fibers in NH3 flow at different temperatures for various lengths of time. The photocatalytic activity and hydrogen production efficiency of the hollow fibers were evaluated under visible-light irradiation. A possible mechanism for the enhanced visible-light photocatalytic activity is proposed.
Experimental
Hollow porous polysulfone fibers with the wall composed of interconnected nanofibers were used as the template material. Fig. 1 shows the schematic preparation steps of N-doped TiO2 hollow fibers. The technique and apparatus for ALD have been reported previously.20–22 TiO2 thin film was coated on the polysulfone hollow fibers by ALD using titanium tetrachloride (TiCl4) and H2O as the precursors for Ti and O, respectively, with the substrate temperature set at 100 °C.23 Each cycle consisted of a TiCl4 precursor pulse of 0.24 s, a purge with N2 for 5 s, followed by a H2O pulse for 0.08 s, and a second purge with N2 for 5 s to remove excess precursor and by-products. The as-deposited TiO2 was amorphous, and therefore the samples were heated at 500 °C for 1 h in air to obtain anatase TiO2 and to remove the polysulfone. The dimensions of the fibers have become smaller after removal of polysulfone and crystallization of TiO2 by the heat treatment. Nitrogen doping was conducted by thermal treatment of TiO2 hollow fibers in NH3 flow (99.9%) at 500–600 °C for 1 h, or at 600 °C for a duration of 1 h to 4 h in a tube furnace.The crystal phases of the specimens were evaluated by X-ray diffraction (XRD, Shimadzu XRD6000) with Cu Kα radiation. The surface morphologies and cross sections of the specimens were examined by field-emission scanning electron microscopy (FESEM, JSE-6500F). X-ray photoelectron spectroscopy (XPS, Ulvac-PHI PHI 1600 ESCA) was performed to analyze the surface chemistry. Transmission electron microscopic (TEM, JEOL-3000F) examination was conducted at 300 kV. The nitrogen content was measured by a N–O analyzer (Horiba, EMGA-620W). Thermogravimetric (TG) analysis at a heating rate of 20 °C min−1 was carried out on a Netzsch STA409 analyzer in air. The specific surface area was estimated by using the Brunauer–Emmett–Teller (BET) method on a TriStar 3000 gas adsorption analyzer with the degassing temperature of 100 °C and the relative pressure of N2 (P/P0) from 0.05 to 0.3. Optical transmittance was recorded on a UV-visible spectrometer (Hitachi 383). The optical property of the specimens was analyzed by photoluminescence (PL) spectroscopy using the 632.8 nm beam of He–Ne laser. The photocatalytic H2 evolution reaction was carried out in a quartz cell using a 150 W Xe lamp as the light source. The visible light was passed through an optical filter, which allowed only wavelengths higher than 420 nm to illuminate the samples. The photocatalyst sample (0.02 g) was immersed into an aqueous methanol solution (5 ml methanol and 20 ml deionized water) under magnetic stirring. Before the irradiation, the reactor was purged with nitrogen for 1 h to remove residual air. The evolved H2 gas was collected and analyzed by a gas chromatograph (Shimadzu GC-2014) at 110 °C using a 5 Å molecular sieve in the column and argon as the carrier gas.
Results and discussion
The shape and morphologies of an individual polysulfone hollow fiber are shown in Fig. 2. The average inner diameter, outer diameter, and thickness of the fiber are 450, 1000, and 275 μm, respectively. The wall is composed of numerous interconnected nanofibers. Fig. 3 shows the SEM images for the cross sections of near middle wall of the TiO2 hollow fibers. Regardless of different treatment temperatures and durations in gaseous NH3, they exhibit nearly identical surface morphologies. The region remains porous and is full of interconnected nanotubes which are formed due to the removal of original interconnected nanofibers within the polysulfone fiber. The structure of TiO2 hollow fibers is also well preserved after treatment in NH3. Therefore, polysulfone is a good framework template for preparation of oxide hollow fibers by ALD technique. A unique characteristic of ALD is that it enables formation of very uniform film with almost atomic precision not only on planar substrates but also on complex three-dimensional porous substrates. As shown by the TEM micrographs in Fig. 4(a) and (b), the interconnected nanotubes within the middle wall of the fiber have the wall thicknesses of 18.7 nm and 18.5 nm, for the samples heated at 600 °C for 1 h and 4 h, respectively. Since the samples were prepared by 400 cycles of ALD, the growth rate is calculated to be approximately 0.46 Å per cycle. From Fig. 4(c) and (d), the lattice fringes indicate that the N-doped TiO2 hollow fibers have good crystallinity. The interplanar distances of 0.348 nm and 0.351 nm could be ascribed to the d-spacing of anatase TiO2 (101), 0.352 nm (JCPDS-ICCD # 21-1272).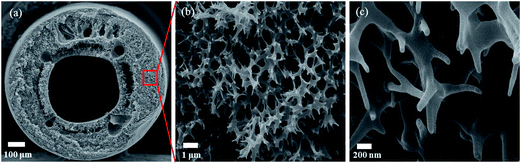 | ||
| Fig. 2 SEM images of a polysulfone fiber. (a) Cross-section, (b) near the middle wall, and (c) higher magnification of (b). | ||
Fig. 5 shows the XRD patterns of TiO2 hollow fibers annealed in NH3 at 500–600 °C for 1 h to 4 h. They all exhibit the anatase phase, and no any other diffraction peaks are observed. The XRD patterns of the (101) plane for the specimens are enlarged in the inset. Basically, it is hard to see shift in the peaks. The d-spacings are calculated and summarized in Table 1. It is seen that the values of d-spacing increase slightly after the nitridation, and they are close to those obtained from the HRTEM analysis. Note that the ionic radii of oxygen and nitrogen are close (1.38 Å vs. 1.46 Å), and the Ti–N bond length, 1.964 Å, is only slightly longer than that of Ti–O, 1.942 Å.24 Therefore, the dimensional change due to N doping is relatively minor.
 | ||
| Fig. 5 XRD patterns of TiO2 hollow fibers annealed in NH3 under various conditions. The inset shows magnified (101) peak of the anatase phase. | ||
| Sample | d-spacing (Å) |
|---|---|
| TiO2 | 3.520 |
| N–TiO2, 500 °C – 1 h | 3.524 |
| N–TiO2, 550 °C – 1 h | 3.523 |
| N–TiO2, 600 °C – 1 h | 3.524 |
| N–TiO2, 600 °C – 2 h | 3.522 |
| N–TiO2, 600 °C – 4 h | 3.525 |
In order to evaluate the proper calcination temperature for removal of polysulfone, a sample was analyzed by TGA during heating to 500 °C (Fig. 6). The polysulfone after coating with TiO2 reveals two stages of weight loss in one hour, as shown in Fig. 6(a). The weight losses from 15 min to 17 min (i.e., 330 °C to 370 °C) and from 17 min to 45 min (i.e., 370 °C to 500 °C) correspond to the partial degradation of sulfone groups and decomposition of polysulfone main chain respectively, leaving only oxide in the fibers. This result implies that polysulfone is completely decomposed at 500 °C after 1 h. Fig. 6(b) shows TG curves versus temperature for pure and TiO2-coated polysulfone. The temperature was kept at 500 °C for 1 h. Both samples are rapidly decomposed at 500 °C.
 | ||
| Fig. 6 (a) TG curves versus time of the polysulfone fiber deposited with TiO2 and (b) TG curves versus temperature for pure and TiO2-coated polysulfone fibers. | ||
Fig. 7(a) shows the absorption spectra of TiO2 hollow fibers annealed in NH3 under various conditions. The TiO2 hollow fibers annealed in NH3 at 500 °C and 550 °C do not absorb visible light. However, with annealing in NH3 at 600 °C for 1 to 4 h, absorption in the visible light region increases substantially. The absorption band edge is shifted to the right and longer wavelength as the annealing time increases. The band gap energies can be calculated by the absorbance data using the equation: αhν = A(hν − Eg)m, where α is absorption coefficient, h is Planck's constant, A is a constant related to the effective masses of electrons and holes, and m = 0.5 for allowed direct transition.25 The band gap energies of pure and N-doped TiO2 hollow fibers are determined to be 3.22 eV, 3.21 eV, 3.19 eV, 3.06 eV, 2.99 eV, and 2.75 eV for the six samples, as shown in Fig. 7(b). Recently, the origins of visible light absorption of C-, N-, and S-doped TiO2 nanomaterials have been studied, and it is shown that additional electronic states above the valence band edge exist, which could define the red-shift absorption of the present materials and band-gap narrowing.26 Other studies have indicated that oxygen sites in TiO2 lattice substituted by nitrogen to form isolated impurity energy levels above the valence band accounts for the visible light response.27 The light absorption of the N-doped TiO2 in the visible light region is important to its practical application since it can be more easily activated by solar light.
 | ||
| Fig. 7 (a) UV-visible absorption spectra and (b) estimation of band gap energies of N-doped TiO2 hollow fibers nitridated under various conditions. | ||
The electronic structure and chemical environment of elements on the surface were investigated by XPS. The surface chemical composition and chemical states of the samples are shown in Fig. 8. For the sample nitridated at 500 °C, no core level N 1s peak is detected, Fig. 8(a). This implies that nitrogen is not incorporated into TiO2. For the sample nitridated at 550 °C, a small peak at a binding energy (BE) of 400.2 eV corresponding to the N 1s core level is detected. Note that the peaks at 400 eV and 402 eV are usually assigned to molecularly chemisorbed γ-N2,28 and some studies have indicated that N 1s peak at 399–400 eV is due to NH3 adsorbed on the surface.7 A peak with the binding energy of 396.4 eV is observed for the samples nitridated at 600 °C, and the peak intensity increases with the treatment time. The peak could be associated with the Ti–N bonding that suggests a substitution of O ions by N ions in the TiO2 crystal lattice.28
 | ||
| Fig. 8 XPS spectra of the N-doped TiO2. (a) N 1s spectra for samples treated in NH3, (b) Ti 2p and (c) O 1s are from the sample treated at 600 °C for 1 h. | ||
Fig. 8(b) shows a Ti 2p XPS spectra of the sample treated at 600 °C for 1 h. Two peaks at 464.8 and 459.3 eV are observed, which can be assigned to Ti 2p1/2 and Ti 2p3/2, respectively. These values agree well with the reported XPS data for N-doped TiO2,29 and can be ascribed to the presence of Ti4+ in the anatase phase. For all samples, the oxygen 1s core level peak appears at the same position, indicating the same nature of oxygen in the structure. However, a small broadening of the O 1s peak is visible in the sample treated at 600 °C for 1 h, as shown in Fig. 8(c). This might be due to the presence of oxygen and nitrogen in the same lattice unit of TiO2. The peak can be deconvoluted into two peaks, one at ∼530 eV and the other at ∼532 eV. The primary peak at 530 eV can be attributed to O–Ti–O type of oxygen coordination, whereas the smaller peak at 532 eV may arise from the presence of N–Ti–O bonds.29
To understand and control the doping of nitrogen in TiO2 hollow fibers, the concentrations of nitrogen in the samples were quantitatively determined by XPS and N–O analyzer, as listed in Table 2. The nitrogen concentration increases with the increase of temperature and annealing time. The values from the N–O analyzer agree well with those from the XPS analysis, except that the analyzer offers one more digit of resolution.
| Sample | XPS | N–O analyzer |
|---|---|---|
| 500 °C, 1 h | 0 | 0.005 |
| 550 °C, 1 h | 0.94 | 0.842 |
| 600 °C, 1 h | 2.37 | 2.563 |
| 600 °C, 2 h | 2.85 | 2.956 |
| 600 °C, 4 h | 4.82 | 4.991 |
The PL emission has been commonly used for investigation of the efficiency of charge carrier trapping, immigration, and transfer behaviors of the photoexcited electron–hole pairs in semiconductors.30 A comparison of the PL emission spectra of pure and N-doped TiO2 is shown in Fig. 9. The PL intensities of the N-doped TiO2 samples are consistently lower than that of pure TiO2, and they are progressively lowered as the nitridation temperature increases. Since the PL emission comes from recombination of excited electrons and holes, the lower PL intensity indicates a lower recombination rate of electron–hole under the light irradiation,31 which may also imply a higher photocatalytic activity of the modified TiO2. This efficient quenching of the PL can be ascribed to two possible pathways: (1) the excited electrons are trapped by the oxygen vacancies, while the holes are trapped by the doped nitrogen, thus the recombination rate of electron-holes is reduced; (2) the excited electrons can transfer from the valence band to the new defect levels introduced by nitrogen doping that exist near the minimum of conduction band.32
Fig. 10 shows the amounts of hydrogen production by P25 and pure and N-doped TiO2 fibers under illumination by a Xe lamp (150 W). The treatment at 500 °C basically does not influence the water splitting efficiency much. The activity gradually increases as the treatment temperature is above 550 °C, and the amount of H2 increases with the irradiation time. After 6 h of irradiation, the amount of hydrogen production for the sample treated at 600 °C for 1 h is 0.185 μmol g−1. The photocatalytic activity of N-doped TiO2 is enhanced because the overpotential of H+/H and the recombination rate of electron–hole pairs are reduced. In addition, the isolated N 2p narrow band slightly above the O 2p valence band was in charge of the visible light response in the N-doped TiO2.27,33 The present result is a direct evidence that N doping narrows the effective band gap of TiO2 and improves the light conversion efficiency in the visible region. It was observed, however, that the amount of H2 decreased with the increase of annealing time at 600 °C. In other words, excess N doping results in decrease in photocatalytic activity. An optimum doping concentration can enhance the photocatalytic performance due to increased separation efficiency of photogenerated electron–hole pairs in the impurity energy levels with an adequate gap so as to inhibit their recombination. On the contrary, excess nitrogen will spread the impurity energy levels which would act as the recombination centers for electron and hole pairs and to decrease the photocatalysis efficiency.27,34
 | ||
| Fig. 10 Hydrogen production of P25, pure and N-doped TiO2 hollow fibers under visible light (λ > 420 nm) irradiation. | ||
A proposed mechanism of water splitting with N-doped TiO2 hollow fibers is illustrated schematically in Fig. 11. When the porous surface of the hollow fibers is irradiated with light, although some of the photons are not directly absorbed by the hollow fibers, they are trapped within the porous structure and then repeatedly reflected until being totally absorbed. Therefore, the porosity can improve the efficiency of photocatalysis.35 Besides, the hollow fibers have a unique structure to entrap incident light inside the hollow structure.36 Such a multiple reflection effect could further improve the collection efficiency of light and elevate photocatalytic performance. The principle of photocatalytic water decomposition of a semiconductor is based on the conversion of light energy into electrical energy on exposure to light. Incorporation of N into the crystalline lattice of TiO2 would modify the electronic band structure of TiO2, leading to formation of an N 2p band at above O 2p valance band. The electrons transfer from the N 2p level directly to the conduction band of TiO2, resulting in higher photoabsorption efficiency. Noted that for hydrogen production electrons are responsible for reducing protons, and the ability of oxidation does not affect the performance because the upper level of valence band of TiO2 is far more positive than the energy level for oxygen evolution. Furthermore, the conduction band remains almost unchanged after N-doping, being more negative than the energy level for hydrogen production.
The present results indicate that nitrogen can be successfully doped into TiO2 by thermal treatment in NH3 that leads to considerable visible light response. The advantages of this nitridation over other methods include (i) use of inexpensive chemical precursors for the synthesis of N-doped TiO2 and (ii) formation of highly porous N-doped TiO2 nanostructure retains the shape and morphology of the polysulfone template. During the photocatalytic reaction, the process in the hollow fibers that possess a large microvoid structure and high fraction of pores should be more dominated by the Knudsen flow, as illustrated in Fig. 11. Knudsen diffusion occurs when the mean free path is relatively long compared to the pore size, so the molecules collide frequently with the pore surface.37 The highly porous structure allows mass transfer of the reactants and products during the reaction. Consequently, Knudsen diffusion caused by the porous confinement enhances the efficiency of photocatalysis. In addition, the highly porous TiO2 hollow fibers containing internal 3D interconnected nanotubes offer a larger reactive surface area (∼100 m2 g−1) for improved photocatalytic activity, compared to commercial P25 TiO2 (50 m2 g−1). It is believed that the main contribution to higher photocatalytic activity of the N-doped TiO2 is band-gap narrowing because of enhanced light absorption in the visible light region. The larger surface area and better light harvesting also enhance the efficiency of photocatalysis, but to a less degree.
Conclusion
Highly porous TiO2 hollow fibers with internal interconnected nanotubes were fabricated by ALD method using polysulfone fibers as a template. Nitrogen doping was achieved by heat treatment in NH3 at above 550 °C. The amount of nitrogen increased with the temperature and duration. Nitrogen was inserted into the anatase TiO2 lattice to form impurity levels at above the valence band that narrowed the band gap to enhance visible light absorption. The highly porous structure of TiO2 hollow fibers possessed a large reactive surface area and better light harvesting that also enhanced the efficiency of photocatalysis. The sample treated at 600 °C for 1 h in NH3 exhibited the highest water splitting efficiency.Acknowledgements
The authors are grateful to Ministry of Science and Technology of Taiwan for supporting this research under the Contract No. NSC 99-2221-E-007-066-MY3 and NSC 101-2120-M-007-013. The polysulfone fibers supplied by Dr Jeng-Liang Guo in Material and Chemical Research Laboratories, Industrial Technology Research Institute, Hsinchu, Taiwan is highly appreciated.References
- A. Fujishima and K. Honda, Nature, 1972, 238, 37 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- A. Di Paola, G. Marci, L. Palmisanok, M. Schiavello, K. Uosaki, S. Ikeda and B. Ohtani, J. Phys. Chem. B, 2002, 106, 637 CrossRef CAS.
- J. Zhu, Z. Deng, F. Chen, J. Zhang, H. Chen, M. Anpo, J. Huang and L. Zhang, Appl. Catal., B, 2006, 62, 329 CrossRef CAS PubMed.
- H. Yamashita, M. Honda, M. Harada, Y. Ichihashi, M. Anpo, T. Hirao, N. Itoh and N. Iwamoto, J. Phys. Chem. B, 1998, 102, 10707 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269 CrossRef CAS PubMed.
- T. Umebayashi, T. Yamaki, H. Itoh and K. Asai, Appl. Phys. Lett., 2002, 81, 454 CrossRef CAS PubMed.
- W. Ho, J. C. Yu and S. Lee, Chem. Commun., 2006, 10, 1115 RSC.
- S. Sato, Chem. Phys. Lett., 1986, 123, 126 CrossRef CAS.
- A. Ghicov, J. M. Macak, H. Tsuchiya, J. Kunze, V. Haeublein, S. Kleber and P. Schmuki, Chem. Phys. Lett., 2006, 419, 426 CrossRef CAS PubMed.
- Y. Suda, H. Kawasaki, T. Ueda and T. Ohshima, Thin Solid Films, 2004, 453–454, 162 CrossRef CAS PubMed.
- O. Diwald, T. L. Thompson, T. Zubkov, E. G. Goralski, S. D. Walck and J. T. Yates, J. Phys. Chem. B, 2004, 108, 6004 CrossRef CAS.
- J. Premkumar, Chem. Mater., 2004, 16, 3980 CrossRef CAS.
- R. W. Sicka, S. H. Rose and T. M. Harkulich, US Pat., 3445361, 1969.
- H. Imai, Y. Iwaya, K. Shimizu and H. Hirashima, Chem. Lett., 2000, 29, 906 CrossRef.
- K. H. Lee and Y. M. Kim, Key Eng. Mater., 1991, 61–62, 17 CAS.
- K. I. Liu, Y. C. Hsueh, H. S. Chen and T. P. Perng, J. Mater. Chem. A, 2014, 2, 5387 CAS.
- D. B. Farmer and R. G. Gordon, Nano Lett., 2006, 6, 699 CrossRef CAS PubMed.
- C. C. Wang, C. C. Kei, Y. W. Yu and T. P. Perng, Nano Lett., 2007, 7, 1566 CrossRef CAS PubMed.
- C. C. Wang, C. C. Kei and T. P. Perng, Nanotechnology, 2011, 22, 365702 CrossRef PubMed.
- W. T. Chang, Y. C. Hsueh, S. H. Huang, K. I. Liu, C. C. Kei and T. P. Perng, J. Mater. Chem. A, 2013, 1, 1987 CAS.
- H. S. Chen, P. H. Chen, S. H. Huang and T. P. Perng, Chem. Commun., 2014, 50, 4379 RSC.
- C. D. Valentin, G. Pacchioni and A. Selloni, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 085116 CrossRef.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627 CrossRef CAS PubMed.
- X. Chen and C. Burda, J. Am. Chem. Soc., 2008, 130, 5018 CrossRef CAS PubMed.
- H. Irie, Y. Watanabe and K. Hashimoto, J. Phys. Chem. B, 2003, 107, 5483 CrossRef CAS.
- M. C. Saha and H. G. Tompkins, J. Appl. Phys., 1999, 72, 3072 CrossRef PubMed.
- C. S. Gopinath, J. Phys. Chem. B, 2006, 110, 7079 CrossRef CAS PubMed.
- Y. Yu, J. C. Yu, C. Y. Chan, Y. K. Che, J. C. Zhao, L. Ding, W. K. Ge and P. K. Wong, Appl. Catal., B, 2005, 61, 1 CrossRef CAS PubMed.
- Y. M. Wu, J. L. Zhang, L. Xiao and F. Chen, Appl. Surf. Sci., 2010, 256, 4260 CrossRef CAS PubMed.
- H. Tang, H. Berger, P. E. Schmid and F. Levy, Solid State Commun., 1993, 87, 847 CrossRef CAS.
- C. Di Valentin, G. Pacchioni, A. Selloni, S. Livraghi and E. Giamello, J. Phys. Chem. B, 2005, 109, 11414 CrossRef CAS PubMed.
- H. H. Chen, F. Lei, J. T. Zhao, Y. Shi and J. J. Xie, J. Mater. Res., 2013, 28, 468 CrossRef CAS.
- B. Lu, X. Li, T. Wang, E. Xie and Z. Xu, J. Mater. Chem. A, 2013, 1, 3900 CAS.
- M. Rahman, F. Tajabadi, L. Shooshtari and N. Taghavinia, ChemPhysChem, 2011, 12, 966 CrossRef CAS PubMed.
- S. M. Mak, B. T. Tey, K. Y. Cheah, W. L. Siew and K. K. Tan, Adsorption, 2009, 15, 507 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |





