Performance of hybrid nanostructured conductive cotton materials as wearable devices: an overview of materials, fabrication, properties and applications
D. P. Hansora†
a,
N. G. Shimpi†
*b and
S. Mishra
a
aUniversity Institute of Chemical Technology, North Maharashtra University, Jalgaon-425001, India
bDepartment of Chemistry, University of Mumbai, Kalina, Santacruz East, Mumbai-400098, Maharashtra, India. E-mail: navin_shimpi@rediffmail.com; Tel: +91-22-26543575
First published on 1st December 2015
Abstract
Smart wearable devices can be fabricated using flexible and linear cable-type materials for applications in energy, electronics, sensing and healthcare products. Such wearable devices have been prepared by incorporating conductive nanostructures, metallic nanomaterials, hybrid nanocomposites and polymer nanocomposites on the surface of flexible and permeable cotton materials (threads, fibers, yarns and fabrics). In this paper, we present an overview of preparation methods of various conductive nanomaterials, hybrids and polymer nanocomposites and their embedment on cotton based flexible materials. The embedment of these functional hybrid nanostructures on the porous and permeable materials has provided the necessary potential for the development of wearable smart devices with improved characteristic properties. Moreover, the diversity of these characteristic properties and potential applications of functionalized cotton materials has been also discussed. This review paper will boost encouragement for the development of next generation smart and flexible devices which could be worn by human beings.
1 Introduction
Flexible and wearable devices have aroused great interest in recent years because of their potential applications in artificial skin, smart electronics, stretchable energy and sensing products as well as advanced biomedical devices. These types of next generation smart systems can be easily worn on the human body, which is soft and curved. Fibrous, flexible and linear textile material based smart devices are ideal candidates for future wearable electronics, which are soft, deformable, washable and durable also. These fibers are transformed into one, two- and three-dimensional (i.e. 1D, 2D and 3D) fiber assemblies. The merging of nanotechnology, electrical, electronic engineering and textile technology has the potential to combine the positive attributes of each technology, the speed and computational capacity of modern smart devices. These are also possible due to their flexibility, wearability and fibrous nature, which are suitable for the fabrication of personal wearable electronics, military garment devices, biomedical and antimicrobial textiles. These research and development activities have been driven by the motivation of fibrous and linear assemblies having different functions of communicating, sensing, computing and actuating etc. The potential for developing conformable, light-weight and flexible electronic devices on textile products is very significant, which offers tremendous opportunities to deploy electronic based energy, sensing devices, built-in or embedded into the cotton based materials.1–13Generally, fabrication of the wearable device requires a linear shape, cable-type, flexible, porous and fibrous material. In the textile industry, cotton is one of the most universal fibrous cloth which is a flexible and a porous material made from threads, fibers, yarns and fabrics. These flexible cotton materials have a complicated structure with high permeability, large surface area and hydrophilic functional groups. These fibrous materials are made of multiple individual weaving micro fibrils bundled together, which contain poly-D glucose chains and have a strong capacity of adsorbing water and other polar solvents. The micro-fibriled cotton materials have strong van der Waals (VdWs) interactions with carbon nanotubes (CNTs) which can be easily coated on the surface of flexible and linear cotton materials via its simple immersion in a solution of CNT ink.1–3,5 Such nanocarbon incorporated hierarchical network creates highly porous and conductive surface morphology, which is essential requirements for an ideal sensor,4,6,12,14–18 energy devices,3,9,10,19,20 supercapacitors (SCs),3,5,7,21–26 lithium ion batteries (LIBs),13,27 flexible electronics,7,8,10,11,28–33 wearable heaters,33,34 human stress detection,18 biomedical devices1,35,36 and solar cells.37 Especially, macroscopic linear shape and excellent mechanical flexibility of such material are also valuable for the development of cable-type devices.5 Similarly, various nanomaterials (NMs), nanoparticles (NPs), hybrid nanocomposites, polymer nanocomposites and flexible materials have shown their immense interest in wearable applications as energy devices,3,5,7,9,10,19,20,22,23,26,27,37 flexible electronic devices,7,8,11,29,30 wearable heaters,33,34 human stress detection devices18 and biomedical devices.1,35,36
This article reviews the current status and recent advances of the next generation wearable devices. In addition, this review paper also elaborates the performance requirements of the flexible and linear cotton material based wearable products, especially regarding the connection between materials, fibrous structures and electronic as well as mechanical functionalities. An attempt is also made to review critically the numerous research publications, which will demonstrate new devices with respect to practicality and manufacturability and also their actual implementation for the development of flexible, wearable and smart devices. This entire review article is divided into four major sections. In Section 1, we introduce and discuss about motivation from past research, while in Section 2 we discuss about various conductive fillers (NMs, nanocomposites, hybrids and polymer composites) along with their fabrication techniques, properties, advantages and disadvantages. In Section 3, we discuss about fabrication and characteristic properties of functionalized and nanostructured cotton materials (threads, fibers, yarns and fabrics). In Section 4, the potential of flexible, smart devices (Fig. 1) based on hybrid nanostructured flexible and linear cotton materials (threads, fibers, yarns and fabrics) have been discussed, which include their wearable applications in the areas of e-textiles, electronics, displays, sensing, environmental monitoring, human body movement, health care appliances, energy conversion, management and storage as well as other important stretchable devices. Together, summaries and perspectives on the future development trends are discussed in the last part. The recent advances in the development of wearable devices and smart textiles will bring this future devices into actual realization, so they could be easily worn by human beings.
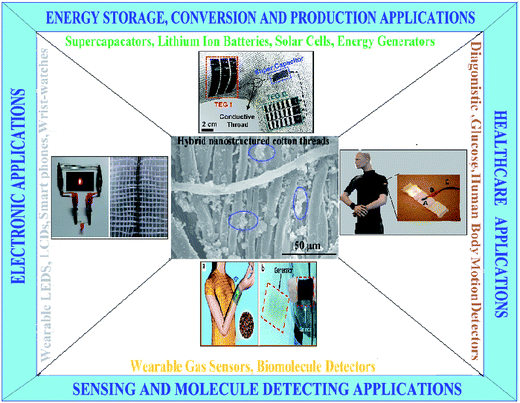 | ||
| Fig. 1 Graphical representation of the main content of this review, illustrates the potential of hybrid nanostructured cotton materials for wearable smart devices in different areas of applications. | ||
2 Conductive fillers for flexible and linear conductive cotton materials
A choice of required materials is an important consideration for the development of wearable devices and the possibility to confer characteristic properties into the flexible and stretchable devices. The development of soft, flexible, semi-conductive or conductive material is essential for the smart and wearable devices because of their unique chemical, mechanical and electronic, properties. Most widely used materials are metals and metal oxide NPs, nanowires (NWs), conductive polymers (CPs), carbon nanomaterials (CNMs) such as carbon particles, carbon fiber, CNTs and graphene, which have been studied. Wearable devices made from conducting materials exhibit good mobility, higher field-effect, steep sub threshold slope, leading to lower operating voltages. These promising materials have also demonstrated their potential in applications including flexible electronics, sensing and energy devices.1–8,10–12,20,26,28,32,34,38–46 Tao et al.11 reviewed and summarized various conducting materials like CPs, CNMs, metallic NPs and NWs. Fig. 2 shows cartoon of the various materials required for the development of a variety of wearable devices and smart textiles. Table 1 shows various CNMs, metallic and semiconducting NMs, nanocomposites, hybrids, CPs and polymer nanocomposites, along with their performance, method of fabrication, advantages, disadvantages and applications.| Structure of cotton materials | Name of material to coat cotton materials | Merits conductive cotton materials | Technique used to coat cotton materials | Advantages of technique | Disadvantages of technique | Electrical property | Wearable application |
|---|---|---|---|---|---|---|---|
| CNMs | CNT/PSS-water/cotton yarn,1,2,7,8,13 CNT/cotton threads,2–4,13,27,32,34,35,93,115 CNT/cotton fabric,3,10–13,28,30,43 CNT/cotton fiber,11,13,16 carbon black/cotton,12 stretchable CNT rope,67 CNT/nylon fibers,74 CNTs/cotton lawn or twill106 | High conductivity and mechanical strength, flexibility, durability, biological compatibility | Dipping and coating,1,2 dyeing,3 wet spinning, screen printing106 | Easy and simple method, long length of conductive cotton yarns, fibers and threads | Slow process | 20 Ω cm−1,1,2 5–125 S cm−1,3 7.8 kΩ cm−1,4 5–50 kΩ cm−1,12 5 kΩ,34 10–3000 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111,115 111,115 |
Biomonitoring and telemedicine and glucose sensor,1,2 energy textiles,3 ammonia sensors,4 heaters,33,34 flexible energy storage106 |
| Cotton–rGO–CNT@CMC,7,20 cotton/graphene,20 fibers of GO,20,73 CF/rGO,22 cotton/carbon–GO,17,23 graphene/silk/PT,36 graphene115 | Conductive and high strength | Wet spinning,7 electrostatic self assembly, one-step electrophoretic method23 | Easily attachable to cotton materials | Multi-process steps, wrinkles on cotton surface | 250 S cm−1,20,73 0.58 S cm−1,36 10–20 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
Energy textiles for SC,7,20 FFSC23 | |
| Metal NPs | Al-coating115 | Stable, stretchable, conductive, with unique geometry, outstanding electronic/optoelectronic properties, excellent mechanical flexibility and good transparency | Chemical solution | Low cost, direct deposition | Catalyst required for process | 19 mΩ cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
— |
| Ag-NPs, Ag-NWs115 | Wet spinning | Simple process | Suitable solvent require | 2200–5400 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
— | ||
| Au, Ni coating,11 cotton/Au-NWs33 | Dip-coating | Simple process, no vacuum required | Annealing is required | 6 S cm−1,11 11 Ω cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
Economical, functional, stretchable, linear and flexible heaters33 | ||
| MnO2/CNT/sponge,19,21 MnO2 nanostructures5 | Dipping–dyeing and electrochemical deposition method | Simple | — | 1800 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
Supercapacitor19,21 | ||
| Cotton/ZnO-NPs45 | Bleaching, mixing with NP solution, solvent evaporation | UV blocking effect required | — | Multifunctional, antibacterial textiles45 | |||
| Lead zirconate titanate/cotton fabric12 | Piezoelectric cantilevers | Screen printing | — | — | — | Piezoelectric cantilevers, (force sensors), energy harvesters and resonators | |
| Hybrids, nanocomposite, polymer nanocomposites | Graphene/textile/Pt-NPs36 | Electrically conductive biomaterial, sensitivity of 0.56 mA mM−1 and LOD 0.2 μM for hydrogen peroxide | Mixed suspension, chemical reduction, electrochemical deposition | — | — | <90 Ω cm−1 | Glucose biosensor36 |
| Cotton/CNT/PPy/MnO2 5 | 3D cable type, flexible, high performance, light weight, foldable, wearable, energy storage | Dipping–coating, electrochemical deposition | Reproducible | Multi steps are required | <20 Ω cm−1 | Wearable cable type super capacitor5 | |
| Cotton/PPy5,97,115 | Chemical and environment stable, thermally stable,97 shielding effectiveness value of −43.9 dB with >99.9 attentuation97 | Functionalization using dopant, in situ polymerization97 | Easy, novel process, high yield | Optimization of dopant | 120–130 S cm−1,115 4.3 × 10−3 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
Super capacitor,5 multilayered shields as microwave absorbing material97 | |
| PAni-NFs,11 PAni-NWs/PVA/CNT/cotton,25 PAni115 | Cost effective | Wet spinning, in situ polymerization11,25,115 | Thick fibers with mechanical strength and electronic properties | Fibers are required to combine | 140–750 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 115 115 |
Two ply yarn SCs25 | |
| Cotton/PEDOT:PSS16,20,32,37,115 | High conductivity, chemically and thermally stable | Dip coating,37 dry spinning,115 soaking16,20,32 | Simple process | Slow coating process | 0.4–2 S cm−1,115 electrical resistance of 430 Ω cm−1,16,20,32 conductivity of 100–110 S cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16,20,32,37 16,20,32,37 |
DSSC37 | |
| PEDOT/tosylate/Au-NPs/cotton fiber,12 PEDOT coated cotton fiber,12 PEDOT:PSS/CNT coated cotton fiber,16 cotton fiber/PEDOT:PSS32 | High conductivity, −20 to 5 gauge factor | Vapor deposition,12 ink-jet printing, soaking16,32 | Coating without using any electrolyte16 | Electrolyte is required, high temperature is required, surfactant is required, metal wire is required | 25 S cm−1,12 430 Ω cm−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
Sensor,12 organic electrochemical transistor for liquid electrolyte, saline (NaCl) sensing,16 human stress monitoring OECT for biosensor32 | |
| CNT/cotton yarns/PVC membrane6 | Conductive, ion-selective yarns, optimum response and selectivity, easily connectable to reading instruments | Dipping–dyeing–rinsing | Target ion immersion required only 15 min. | Protection is required, immersion required long time of 12 hour, heat shrink tube is required | 500 Ω cm−1 | Electrochemical sensors for wearable devices for detection of pH, K+ and NH4+ | |
| Cotton/CNT/PTFE8 | Measures physiological and biomechanical signals, human body motions | Dipping–dyeing- | Simple, cost effective, home-made dipping and drying steps | High performance polymer is required for coating of cotton | 0.644 kΩ cm−1 | FBG device for wireless body temperature measurement8 | |
| PPy/Lycra/cotton fabrics11 | Resistive fabric, detect human body posture and gesture | Printing on fabrics | — | — | Gauge factor 80, 50% strain, | Pressure and strain sensor11 | |
| CNT/polyester/cotton12 | Piezo-, thermo-, magneto-, chemo- and photo-resistive | Doping | Metal and organic particles can be doped | — | 125 S cm−1 | Sensing applications12 | |
| PAni-cotton12 | High conductivity | Oxidative polymerization | — | Oxidant is required | 3 Ω cm−2 | — | |
| Low cost wax patterned cotton cloth,17,93 cotton fabric/carbon graphite35 | Improved wicking property | Wax patterning method17,35,93 | Simple | Scouring of cloth is required, computer software required to pattern the templates | — | Colorimetric bioassays, 2D, 3D microfluidic devices to detect BSA17 FED to detect lactate, hydrogen peroxide,35 ELISA as diagnosis device93 | |
| Cotton threads/CNTs/PAni-Fe2O3 123 | Quick response time and maximum response value | Ultrasound assisted coating and oxidative polymerization | Effective dispersion of NPs, NMs, uniform distribution | — | — | LPG sensing at room temperature |
2.1 Types of materials
Graphene is a unique and attractive energy material because of its one atom-thick 2D structure which makes it easily deformable in the direction normal to its surface, providing good mechanical flexibility. The carbon–carbon σ bond is the strongest single bond in nature, endowing graphene with high Young's modulus and tensile strength. The inherent mechanical and electronic properties of graphene make it an attractive material for the applications in bendable, foldable, stretchable and/or flexible photovoltaic devices, fuel cells, nano generators, SCs, LIBs and other devices related to energy conversion such as organic light emitting diodes (LEDs), photodetectors and actuators. Thus, graphene sheets, especially chemically modified graphene, such as GO and rGO can also be assembled into various macroscopic flexible materials such as fibers, thin films, and 3D porous networks. Recently, Shi et al.20 reviewed and discussed about various flexible graphene devices for energy conversion and storage applications. They also summarized various graphene materials used for flexible optoelectronic devices, SCs and LIBs.20 Furthermore, graphene sheets and their derivatives are frequently blended with polymers or inorganic NPs to improve the flexibility of the resulting composites and/or extend their functions.20,22,23,36,40–43,53,56,71–76
Semiconducting metal oxides, solid electrolytes, ionic membranes and organic semiconductors seem to be the classical materials for sensing devices,52,84,85 e.g., chemical vapor deposition (CVD) grown SnO2-αFe2O3 multilayers.86 The WO2.72-NWs84 and α-Fe2O3-CNTs85 for detection of H2S, nanostructures of Fe2O3 as sensing layers for detection of liquefied petroleum gas (LPG) at room temperature,77 ultrasonically atomized hollow spheres of SnO2 nanostructures for detection of 1000 ppm LPG at the 350 °C and adsorption of LPG as well as oxygen on nanostructured Fe2O3 hollow spheres.87 Metallic (Au,61 Pt,36 Ag33) NPs, NWs and nanostructures of various metal oxides and dioxides (MnO2,5 ZnO,78 TiO2,79 WO2,84 Fe2O3,77,85,87 SnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 87), CNT and graphene1–8,10–13,16,17,20,23,27,28,33–35,38,52,53,56,60,63,64,67–69,71,74,78,80,93 have been also reported in the development of cotton/textile based wearable devices. Recently, Shen et al.30 reported various metallic NWs (Cu, Au and Pt) and semiconducting oxide NWs (ZnO, CdO, ITO, SnO2, In2O3, Cu2O, Fe2O3 and V2O5) for the development of flexible electronic devices. Recently, Algar et al.81 reported various NPs for diagnostic with consumer electronic devices.
87), CNT and graphene1–8,10–13,16,17,20,23,27,28,33–35,38,52,53,56,60,63,64,67–69,71,74,78,80,93 have been also reported in the development of cotton/textile based wearable devices. Recently, Shen et al.30 reported various metallic NWs (Cu, Au and Pt) and semiconducting oxide NWs (ZnO, CdO, ITO, SnO2, In2O3, Cu2O, Fe2O3 and V2O5) for the development of flexible electronic devices. Recently, Algar et al.81 reported various NPs for diagnostic with consumer electronic devices.
Novel nanostructures of CNT–metal hybrid clusters (synthesized by infiltrating SWCNTs with the transition metals Au, Ag, Ti, Mn, Fe, Co, Ni, Pd or Pt) have shown their specificity of adsorbing the various gas molecules, e.g. CNT–Al cluster nanosystem for detection of NH3 molecules,14 MWCNTs-(SnO2, TiO2 and CuO) NPs based hybrids for H2S52 vapor sensing, Ni-CNT hybrid base active sensing layer for detection of ethanol59 vapor up to 250 ppm at room temperature, hybrids of CNT–Au, CNT–Pd, CNT–Ag for detection of 100 ppm NH3, 50 ppm CO, 5000 ppm CO2 and 100 ppm ethanol60 and hybrids of Au-NPs mats decorated with CNT for detection of pollutants like NO2, CO and C6H6.61 The sensing performance (sensitivity, selectivity and response time) of CNTs-metal hybrids can be improved by rational functionalization of their surface with different materials (decorating with metal oxide NPs or by grafting functional groups) by different methods (covalent and non-covalent).52–65 Ab initio study of doped CNT based sensor has been analyzed for a new breed of sensing devices demonstrating the viability of vapor (e.g. NO2, NH3 and O2) detection. Externally functionalized CNT and internally doped CNT may result in temporary sensing capability due to the weak VdWs interaction between CNT and doped materials. Besides this, researchers have also developed the nanostructured hybrid sensors for detection of CO and water molecules.58 Chemi-resistive sensor arrays have been developed using SWCNTs covalently functionalized by urea and thiourea66 for detection of cyclohexanone and nitro methane. Hybrids of chitosan and chitosan-co-poly(ε-caprolactone) grafted/functionalized MWCNTs were also reported for vapor sensing of organic compounds (ethanol, methanol toluene, and chloroform).88 Chitosan-functionalized GO hybrid nanosheets have shown their potential for effective for drug-delivery.89
CP based nanocomposites were fabricated by embedding the metal oxide and metal sulphide NPs, which have demonstrated their sensing performance, dependable on their electrical characteristics.82,83 Considering the growing interest towards electrically conductive hybrid nanocomposites, camphor sulfonic acid doped PAni-ZnO nano-sensor performed well with maximum response up to 28.11% for the detection of 100 ppm NH3 at room temperature.87 Polyaniline nanofibers (PAni-NFs) having a diameter of 60–100 nm possess a high conductivity (120–130 S cm−1) at room temperature.11 Moreover, the strategies of preparing the ternary CNT/PAni composites consolidating noble metal NPs, metal oxide, or graphene sheets are found suitable in potential applications such as chemical sensors, capacitors, fuel cells and electronic devices.68 A p-PAni/n-TiO2 heterojunction based sensor can give maximum response of 63% on exposure of 0.1 vol% LPG at a room temperature.79 A blend of two 1D NMs (e.g. hybrid nanocomposites of CNT/PAni-NPs and core/shell polymer nanocomposites of CNT/PAni) has been reported with incredible interest towards higher sensitivity to NH3.53 The nanocomposite films of PAni-MWCNT-gold hybrid system exhibited great electrocatalytic and sensing actions toward detection of ascorbic acid. A sensor based on PAni–prussian blue–MWCNT hybrid composite has been developed for the detection of 0.01 mM glucose and H2O2 glucose.69 The PAni–CNT composites have also been studied for the detection of CO2, nitrite, glucose, acetaminophen in acetate as well as pH sensor.79,80 CNT/PAni nanocomposites have shown better thermal stability, electrical response and higher sensitivity towards NH3, H2S, acetic acid, hydrazine (N2H4) detection, industrial monitoring, personal safety and medical field.80 The CNTs were decorated by chitosan/CP composites to form nanohybrid materials for the detection of polar vapors. Poly(ethylene oxide) (PEO) and poly(vinyl alcohol) (PVA)–CNTs nanocomposites base sensors were introduced for detecting organic vapors of methanol, ethanol, isopropyl alcohol, chloroform and water.15,88 The chemical sensors based on CNTs/polymer or graphene/polymer nanocomposites are also reported for quantitative and qualitative analysis in diverse application fields of bio-sensing (enzymes, proteins, antigens and metabolites), gas and chemical sensing using electrochemical and optical detection methods.71
2.2 Fabrication process
2.2.1.1 Carbon nanostructures. In 1991, Iijima et al.98 developed a CNT as an intriguing form of a pure carbon graphene sheet which can be rolled into a nanotubular shape and their both ends capped with a half of fullerene molecule. After the discovery of CNTs, various synthesis methods such as CVD, arc-discharge, and laser vaporization have been reported. Amongst all these methods, CVD has been emerged as an effective and suitable method for large production. In this method catalyst disintegrates the hydrocarbon atoms, which affects the growth mechanism, morphology, diameter, type of CNTs and their properties.38,39,48–50,99
The CVD seems to be the most effective method for CNT's better growth and development which are affected by methods of nanocatalyst preparation, nature and pore size of the support and metal, the quantity of active catalyst NPs and size distribution of the active component. Using the CVD method, CNTs can be synthesized on metal nitrate phases as precursors with different support materials and gases by varying temperature.49 The MgO is the most promising support material due to presence of large numbers of alkakine reacting sites. Most common methods like impregnation, sol–gel and combustion technique have been used for the preparation of nanocatalyst which is required for proper growth of the CNT. The yield of CNTs can be increased by changing the gas composition of hydrocarbon gas during the CVD growth of SWCNTs and MWCNTs over metal-supported MgO and Fe catalysts.94,95 The transition metals such as Cr, Mo, Fe, Co and Ni are the suitable nanocatalysts for the decomposition of hydrocarbon and proper formation of the CNTs. The vicinity of hydrogen promotes decomposition of carbon as well as the addition of co-catalysts such as metal (Cu, Sn, K) or a non-metal. Catalyst composition and drying process are other main factors that affect the properties and productivity of CNTs.97 Vertically aligned catalyst free CNTs with high efficiency and carbon purity of 99.95% can be synthesized by water-assisted CVD with 85% selectivity and the catalyst film thickness of sputtered Fe.97 A purification of CNTs is required because as-grown CNTs sample coexists with other carbon species such as amorphous carbons, carbon nanostructures and transition metals used as a catalyst NPs. The CNTs can be purified by combining wet grinding, hydrothermal treatment and oxidation processes by refluxing raw CNTs in nitric acid to oxidize metals and unwanted carbons followed by oxidation in air at 550 °C for 30 min to obtain >98 wt% pure SWCNTs. Later, purification procedure was improved and the three-step purification method was developed that includes another vacuum annealing at 1500 °C to recorder the tubes and yielded 99.9% pure SWCNTs with respect to metal content.49,99–101 He and Yun et al.44 synthesized SWCNT films by CVD with ferrocene and xylene as a catalyst and carbon precursors at a reaction temperature of 1160 °C. The carbon precursor in catalyst solution (0.045 g mL−1) with a small addition of sulfur (0.001 g mL−1) was injected into the CVD furnace at a rate of 10 μL min−1 and carried by a gas mixture (Ar/H2, volume ratio 0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15) at 1500 cm3 cm−1 into the reaction zone, typically for a period of 30 min. An entangled yarn consisting of many double-helix segments were prepared using as-synthesized freestanding CNT film (15 cm in length) by suspending them horizontally with two ends fixed on an electric motor and a metal block, respectively.44 Stretchable and long CNTs can be freely grown on Inconel substrate without using any external catalyst in microwave plasma CVD.102
0.15) at 1500 cm3 cm−1 into the reaction zone, typically for a period of 30 min. An entangled yarn consisting of many double-helix segments were prepared using as-synthesized freestanding CNT film (15 cm in length) by suspending them horizontally with two ends fixed on an electric motor and a metal block, respectively.44 Stretchable and long CNTs can be freely grown on Inconel substrate without using any external catalyst in microwave plasma CVD.102
On the other hand, various methods have been developed to synthesize single or few-layer graphene sheets.75 Among them, mechanical exfoliation of graphite with scotch tape was first employed and led to the discovery of graphene. This method can produce high-quality graphene sheets with smaller sizes in low yield for fundamental researches. Oxidative exfoliation of natural graphite to GO followed by the reduction of GO is one of the most efficient method for low-cost and large-scale production of single-layer graphene. Direct growth of graphene via CVD is the most promising technique to produce large-area graphene sheets. To fabricate flexible devices, CVD grown graphene sheets have to be transferred from the surfaces of catalytic metal films onto flexible target substrates. Although the formed GFs have high electrical conductivity (∼10 S cm−1), but cannot be produced continuously with desired lengths and strength on a large scale.113 Qu et al.113 reviewed and reported various GF fabrication methods such as spinning of GO, hydrothermal strategy, CVD, spontaneous reduction and assembly of GO, graphene yarns from aligned CNTs, electroforetic self assembly, self assembled GFs. These GFs have electrical conductivity of 10–280 S cm−1, mechanical strength 33–442 MPa and Youngs's modulus of 2.8–39.9 GPa.113 Flexible graphene thin films can also be prepared by the deposition of multilayer GO/rGO sheets onto various substrates by spin coating, spray coating, dip coating or electrophoretic deposition. A free-standing paper like GO film can be prepared by vacuum filtration. Earlier, GO was synthesized by a modified Hummer's method using heating a solution of raw graphite powder, K2S2O8, P2O5, H2SO4. Subsequently, this reacting solution was filtered with de-ionized (DI) water, followed by drying in air and oxidation in H2SO4/KMnO4/DI water/H2O2 solution. The oxidation end of the solution was confirmed by color change to light brown. The GO solution was washed, filtered with HCl and then redispersed in DI water that had been dialysed for 2 weeks.23,33,113
Inspired by CFs and CNT yarns, the fabrication and applications of graphene-based 1D fibers have shown immense interest in assembled form of 2D flexible materials such as papers, and conductive transparent membranes. Graphene fibers (GFs) possess characteristic properties of fibers like light weight, ease of functionalization, mechanical flexibility for textiles, good electrical conductivity. Most GFs were prepared from their GO precursors by wet-spinning of liquid crystalline GO dispersion.20,73,113 Gao et al.73 reported a method of spinning process for GO fibers, these GO dispersions were injected using glass syringes into the NaOH/methanol solution at 1.5 MPa of N2 atmosphere. This GO dispersion was continuously injected into a coagulation bath to form fibers with tunable diameters (50–100 μm) and several meters long fibers as shown in Fig. 3(a–c). The fibers in the coagulation bath were rolled onto the drum, washed with methanol to remove the salt and dried for 24 h under room temperature. The chemically converted GF were prepared by chemical reduction of as-prepared GO fibers in the aqueous solution of hydroiodic acid at 80 °C for 8 h, followed by washing with methanol and vacuum drying for 12 h. After chemical reduction, GO fibers were converted into rGO fibers having good mechanical properties (tensile strength up to 180 MPa and an elongation at break of 3–6%) and high conductivity (∼2.5 × 102 S cm−1). Notably, these GFs are flexible; can be fastened into tight knots without any breakage, or integrated into conductive patterned cotton textiles with other threads as shown in Fig. 3(d–f). Hydrothermally reduction of a GO suspension, sealed in a long and thin tube can also produce conductive rGO fibers. The strong and flexible GFs are wearable and shapeable and can be woven into engineered structures. Hollow GFs can also be prepared by template guided assembling rGO sheets in a tube via a similar hydrothermal process. One removable Cu metal wire was placed in a glass pipeline and the hydrothermally prepared rGO sheets were assembled around the wire to form a compact skin. The as-prepared hollow GFs are mechanically stable and flexible, can be shaped to a specific geometry, i.e. graphene-based flexible 3D porous architectures.20,73,113
 | ||
Fig. 3 SEM image of the (a) GO fiber, and its (b and c) typical tighten knots, (d) 4 m long GO fiber wound on a Teflon drum having 2 cm diameter, (e) a chinese character (“ ”, Zhong) pattern knitted in the cotton network (white) using two GF (black), (f) A mat of GF (horizontal) woven together with cotton threads (vertical). Scale bars, (a–c) 50 μm, (d) 2 cm and (e and f) 2 mm. Reprinted with permission from ref. 73. ”, Zhong) pattern knitted in the cotton network (white) using two GF (black), (f) A mat of GF (horizontal) woven together with cotton threads (vertical). Scale bars, (a–c) 50 μm, (d) 2 cm and (e and f) 2 mm. Reprinted with permission from ref. 73. | ||
2.2.1.2 Metal nanoparticles. Among the nano sized metal oxides that have been focused due to their potential research and majority application in electronic devices. Recently, Shen et al.30 also discussed various synthesis methods for preparation of metallic NWs and semiconducting oxide NWs. Most widely reported methods are hydrothermal, solvothermal, reflux, microwave synthesis, vapor–solid (VS) and vapor–liquid–solid (VLS) techniques, electron beam lithography, photo-lithography.30 Complex structures of nano sized γ-Fe2O3 are being widely used, which can be synthesized via various routes like hydrothermal, microwave, flame spray pyrolysis, solvothermal and sonochemical method. The γ-Fe2O3 nanostructures were prepared by sonochemical route, which can serve as sensing layers for detection of gases, volatile organic compounds (VOCs) and other agents.77 The NPs of SnO2, CuO and TiO2 have been prepared using ultrasonic bath and appropriate metal chloride salt.52,79 Sonochemically synthesized metal silver and cadmium (Ag and Cd) sulphide NPs have shown their potential for gas sensing application.82,83 The NPs of WO3 have been prepared by hydrothermal method, while WO3-NWs were prepared by solvothermal synthesis.84 Several methods such as a template method, a sol–gel strategy, gas–solid reaction techniques and a hydrothermal approach, have been developed for the synthesis of 1D nanostructures of α-Fe2O3.85 Nanostructured hollow spheres of SnO2 with fine Fe2O3-NPs were synthesized by ultrasonic atomization. Different surface modified films (Fe2O3 modified SnO2) were prepared by dipping them into 0.01 M aqueous solution of ferric chloride at regular intervals of time followed by firing at 500 °C.87
2.2.1.3 Hybrid nanocomposites. Metal-NPs can be functionalized on CNTs to form hybrid nanocomposites, which enable an attachment of other species. Indeed, hybrid nanocomposites of CNTs coated with Pd-NPs have been successfully synthesized. Methods for creating and dispersing metal clusters are also well known and coating of CNT with metal clusters has already been achieved by electron beam evaporation, chemical attachment of preformed clusters and precipitation from metal salt solution.14,89 A sputtering technique has been implemented to deposit platinum (Pt), and gold (Au) nanoclusters on CNTs to form hybrid nanocomposites.60 The thermal evaporation route has been also reported in the development of Au–CNT hybrid nanocomposites.61 Rhodium (Rh) and Pt-NPs can be decorated on SWCNTs by electrochemical deposition.64 A novel and simple route has been reported to synthesize α-Fe2O3 nanotubes using CNT as templates via the thermal decomposition.85 The carbon-based nanomaterial films prepared by layer by layer (LbL) hybrid assembly serve as an emerging platform for the preparation of both energy storage electrodes and sensing applications. Char and Kim et al.103 summarized various CNMs based multilayered films prepared by the LbL assembly along with their applications. LbL assembled multilayer structures have potential characteristic properties while maintaining a simple aqueous based process.103 Gao et al.7 proposed a coaxial wet-spinning assembly approach to spin continuously polyelectrolyte-wrapped graphene/CNT core-sheath fibres, which are used directly as safer electrodes to assembly two-ply yarn SC. The coaxial wet-spinning assembly approach was extended to prepare continuous coaxial fibers with the core of mixture of rGO and CNTs, denoted as rGO-CNT@CMC. The combination of scalable coaxial wet-spinning technology and excellent performance of yarn SC paves the way for wearable and safe electronics.7 Ye et al.36 prepared a freestanding graphene–silk composite film via vacuum filtration of GO and silk fibers mixed suspension, followed by chemical reduction. Spiky structured Pt nanospheres (700 nm) were grown on these graphene/silk composites film substrate by cyclic voltammetry electrodeposition. Using these Pt-decorated graphene/silk composites, a glucose biosensor electrode was fabricated by enzyme immobilization.36
Hong et al.103 prepared an rGO/CNT hybrid composite paper by casting the mixed dispersion of GO and functionalized CNTs followed by their thermal reduction. This hybrid paper was mechanically stable and it can recover to its original shape by releasing from its twisted or bent state. For the applications as flexible, transparent conducting electrodes, CVD-graphene is a better choice than rGO sheets as the starting material for preparing graphene/CNT based hybrid composites.71 A transparent, flexible graphene/CNT membrane was fabricated by covering a CVD-graphene sheet onto the surface of a large-area CNT thin film. Flexible composites of graphene and inorganic-NPs are widely employed in portable and wearable devices, such as solar cells, LED, fuel cells, SCs, LIBs and sensors. A variety of inorganic nanostructures have been blended with graphene and their derivatives, including metals (Pt, Pd, Ag, Si, Cu), metal oxides (RuO2, MnO2, V2O5, Mn3O4, Co3O4, SnO2, TiO2, NiO, Fe3O4, ZnO and BaTiO3), metallic compounds (InN and CdS; CdSe) and, bimetallic hybrids (Al–TiO2, Fe2O3–SnO2, Au–Pt and Cu–Ag). Ex situ and in situ hybridization methods are used to synthesize these types of composites. Interestingly, 2D array of Au-NPs at oil–water interfaces could be transferred onto flexible GO paper by dip coating, forming a monolayer of densely packed gold NPs on GO paper. A similar approach has also been used to assemble core–shell (Au–Pt) NPs on flexible rGO paper. A flexible N-doped graphene/SnO2 composite based paper was obtained by hydrothermal treatment of the mixed solution of SnCl4·5H2O and GO followed by vacuum filtrating of the reaction solution. In addition, direct electrochemical deposition is an attractive approach to load MnO2-NPs on a flexible CVD grown graphene network. This 3D hybrid network of MnO2/graphene was bendable and foldable, little change (less than 1%) in electrical resistance was observed after bending for 500 cycles to different angles.20,33
3 Functionalized and nanostructured flexible and linear conducting cotton materials
Wearable devices have revealed numerous brilliant and smart designs showing valuable gimmicks like adaptability, light weight and foldability. Various hybrid nanostructured flexible and linear conductive cotton materials are discussed here with the end goal being able to attain wearable gadgets in the field of energy conversion and storage sensing, displays and presentations, (e.g. sensing gadgets, embedded vital signs monitoring devices, and convenient cum portable power gadgets). Solid VdW interactions of CNTs with these sorts of poly-D glucose chains of flexible cotton materials, can make them profoundly conductive without influencing their shape. When the CNT get adsorbed on cotton material and then dried, it is difficult to expel the adsorbed CNTs from the filaments by exposure to solvents, heat, or a combination of both. The incorporation of CNTs on the surface of cotton materials was accounted much more efficient than their adsorption into carbon fibers.2 Such a hierarchical network creates a confounded and porous surface morphology with high conductivity, which meets the necessities of a perfect wearable technology. The permeable structure allows high mass stacking of dynamic materials, which could further increase the sensing,2,4,6,12,14,16,34,38,40,42,44,48,50–53,55–64,70,75,78,79,86,88,97 electronics,2,7,8,11,12,29,30,38,41,48,50,74,75,91 biomedical1,2,9,17,28,32,35,36,38,48,50,75,81 and energy storage capability.2,3,5,10,12,13,19,20,22,23,26,27,30,38,42,48,503.1 Fabrication process
Conductive textiles can be fabricated by depositing a thin layer of metal on the textile surface via “galvanic deposition”, “atomic layer deposition”, “electrochemical deposition”, “electroless deposition” (ELD) or EDM and others. Among these techniques, ELD is particularly attractive, because it does not require expensive equipment and can be carried out under ambient conditions at a large scale.117 Tao et al.11 and Lee et al.115 reviewed various fabrication methods, surface mounting technology, conductive nanotechnologies and self-organizing technologies because fabrication methods play an important role in determining the characteristics, cost, and stability of the fiber-based flexible and wearable electronics and energy devices.11,115 Castano and Flatau et al.12 reviewed and reported various materials, connections and fabrication methods for smart fabric sensors and e-textile technologies. He reported that the conductivity of cotton materials can be enabled during and after manufacturing. Extruded threads, twisted wires, and fibre/yarn coating are examples of conductivity enabled textiles during their manufacturing, while conductive fillers such as ICPs, CPs, CNMs, NPs, NMs, NWs are used to coat flexible and linear cotton materials by “dipping and drying”, “film coating”, “screen printing”, “silk screening”, “sputtering”, “electrochemical deposition”, “electroless plating”, “CVD”, “self assembly”, “epitaxial growth”, “chemical reduction”, “pulsed laser deposition” etc.12Majority of coating technique depends greatly on the surface morphology and surface tension of the textile substrate, which can also vary from section to section and may result in non-uniform coatings. An appropriate coating technology should impart the desired functionalities and/or provide a suitable interface layer for high durability. Due to the large surface area-to-volume ratio and high surface energy of NMs, conductive coating with discrete molecules or conductive NMs can bring individually to designated sites on textile materials in a specific orientation and trajectory through thermodynamic, electrostatic or other methods. The key consideration is if one can apply, durable, nano-scaled coatings have been used for textiles in a cost-effective manner while satisfying the requirement of electronic functions. In this regard, low-cost and low-temperature processes without vacuum environment are preferred.11,12 In an e-textile, the conductive lines are established or conductive threads are woven typically by (i) manually attaching conventional wires or sewing conductive thread, (ii) replacing nonconductive fibers with conductive ones, (iii) machine embroidering of conductive cotton thread, (iv) weaving and (v) printing rigid and stretchable conduction lines (i.e. inks and polymers) using microelectronics techniques.11,12 Cheng et al.27 reviewed and summarized about direct growth or deposition/coating of active materials on the porosity and a large exposed surface area of conductive carbon cloth.27 The development of wearable smart devices requires NMs, functional nanocomposites, hybrid nanostructures, polymer nanocomposites and porous, flexible and linear cotton materials. Following are the previously reported preparation methods, routes, approaches, techniques which have been used for the development of hybrid nanoastructured conductive cotton materials.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio. The conductive cotton materials (e.g. threads) can be nanoscopically coated by using the CNT ink through the several time repetitions of “dipping and drying” steps. In these steps, an ink with well-dispersed CNTs is generally prepared by dispersing pure and treated SWCNTs in water with a surfactant (e.g. Sodium dodecyl benzene sulfonate (SDBS)).1–4,6,8 A dispersion of 50 mg CNT, 100 mg SDBS and 10 mL DI water, was prepared by bath sonication for a time of 10–20 min. This CNT ink dispersion can be probe-sonicated for 90 min. Then a cotton thread was dipped into the black CNT ink. Owing to the strong absorption, the cotton threads became tightly coated by CNTs. The cotton thread with SWCNT ink was subsequently dried in oven at 70–80 °C for 120 min.1–8,19,34 Similarly, deposition of Au-NWs films around cotton threads can be achieved through the dip-coating process, reported by Goldthorpe et al.33 A cotton thread (200 mm) was cleaned in an ultrasonic bath using ethanol, alcohol, and DI water for 5 min each. The NW solution adhered well to the surface of the cotton thread. The density of the deposited Au-NWs film was varied by using different concentration of the Au-NWs in the coating solution and the number of dipping steps. After deposition the cotton threads were annealed in air at 150 °C for 30 min. To obtain a stretchable conductive thread, a multifilament polyester/rubber blend (28% polyester, 72% rubber) with a diameter of 450 mm was coated. The thread was stretched to 150% of its original length using a vice, surface modified with NaOH, and then coated with Au-NWs in the stretched state by drop-casting. The coated-thread was then annealed using a heat gun at the 150 °C for 60 min.33
1 weight ratio. The conductive cotton materials (e.g. threads) can be nanoscopically coated by using the CNT ink through the several time repetitions of “dipping and drying” steps. In these steps, an ink with well-dispersed CNTs is generally prepared by dispersing pure and treated SWCNTs in water with a surfactant (e.g. Sodium dodecyl benzene sulfonate (SDBS)).1–4,6,8 A dispersion of 50 mg CNT, 100 mg SDBS and 10 mL DI water, was prepared by bath sonication for a time of 10–20 min. This CNT ink dispersion can be probe-sonicated for 90 min. Then a cotton thread was dipped into the black CNT ink. Owing to the strong absorption, the cotton threads became tightly coated by CNTs. The cotton thread with SWCNT ink was subsequently dried in oven at 70–80 °C for 120 min.1–8,19,34 Similarly, deposition of Au-NWs films around cotton threads can be achieved through the dip-coating process, reported by Goldthorpe et al.33 A cotton thread (200 mm) was cleaned in an ultrasonic bath using ethanol, alcohol, and DI water for 5 min each. The NW solution adhered well to the surface of the cotton thread. The density of the deposited Au-NWs film was varied by using different concentration of the Au-NWs in the coating solution and the number of dipping steps. After deposition the cotton threads were annealed in air at 150 °C for 30 min. To obtain a stretchable conductive thread, a multifilament polyester/rubber blend (28% polyester, 72% rubber) with a diameter of 450 mm was coated. The thread was stretched to 150% of its original length using a vice, surface modified with NaOH, and then coated with Au-NWs in the stretched state by drop-casting. The coated-thread was then annealed using a heat gun at the 150 °C for 60 min.33Multiple individual cotton fibers are composed of multiple micro fibrils bundled together and also having poly-D glucose chains, usually arranged in crystalline, or partially crystalline, domains (Fig. 4(a)). This kind of structure allows absorbing the large amounts of water or other polar solvents, which causes the fibers to swell at a time of dipping in conductive solutions. And also CNTs have been proven to have large VdW interactions. Furthermore, acid treated CNTs have carboxyl functional groups on the surfaces and the ends, which can form strong hydrogen bonds with the hydroxyl groups present in the fibers. Upon contact, large VdW forces and hydrogen bonding occurs, which binds the CNTs very tightly to the cotton materials.3,5 Fig. 4(b and c) shows a schematic of CNTs (1.6 mg mL−1 in water with 10 mg mL−1 SDBS) wrapping around fibers to create a 3D porous structure as shown in Fig. 4(d–g) and highly conductive cotton fabric which also retain their texture and structure after CNT (4 mg mL−1 in water with 8 mg mL−1 SDBS) coating and feel the same as the original material. This fabrication process can be easily applied to other ink made of nanostructured materials and scaled up with roll-to-roll techniques using slot-die or curtain coating processes.3 Scanning electron microscopy (SEM) images of Fig. 4(d and e) reveals the macroporous structures of a cotton fabric sheet. Conformal coating of SWCNTs onto the fibers was observed for the cotton fabric (Fig. 4(f)). This conformal coating is a result of the mechanical flexibility of individual SWCNTs and the strong binding energy between SWCNTs and the cotton fibers that accounts for the high conductivity of the stretchable and porous textile. Tunneling electron microscopy (TEM) images (Fig. 4(g)) taken on SWCNT–cotton fiber hybrids show that the SWCNTs are well bonded to the fiber and forming cross-linked networks, which provide conducting pathways. Such double porous structures facilitate the easy access of electrolyte ions to the SWCNTs, which is an essential requirement for high power SC applications.3 As shown in the schematic in Fig. 4(h), MnO2 was uniformly electrodeposited on the SWCNTs using a solution of 20 mM Mn(NO3)2 and 100 mM NaNO3. Three electrodes namely Ag/AgCl as a reference electrode, platinum foil as a counter electrode, and SWCNT coated cotton fibers as a working electrode were used in the deposition process. An PPy film was coated on conductive (MnO2–CNT nanostructures wrapped) cotton fibers by “electroceposition” with a constant voltage of −0.8 V using a similar method using a solution of 0.2 M NaClO4 and 5 vol% pyrrole monomer.5 The open structure of cotton allows excellent deposition of the MnO2 conformally along the fibers (Fig. 4(i)). The deposition is observed on the surfaces of the SWCNT/cotton thread as well as inside the layers of cotton fibers. The conductive cotton fibers were peeled apart and SEM was taken of the interior cotton (Fig. 4(i)). Conformal coating of MnO2 on the cotton fiber's surface is clearly observed and the peeling leads to the partial delamination of MnO2 from the cotton fibers as shown in Fig. 4(i) which also reveals the flower structure of MnO2 particles deposited on the SWCNT surfaces. Hierarchical network of CNT coated cotton threads creates a highly porous surface morphology with excellent mechanical flexibility and high conductivity, which meets the requirements for an ideal platform of cable-type SC devices.5 As shown in Fig. 4(j and k), a highly conductive and porous MnO2/CNT/cotton sponge based hybrid electrode can be prepared through the coating of CNTs by a simple and scalable “dipping and drying” method, followed by “electrochemical deposition” of MnO2.19,21 The fabrication process consisted of four simple steps, as illustrated in Fig. 4(j). A piece of commercially available sponge (pore sizes 100 × 500 μm) was cleaned with water and acetone several times. After drying completely in a vacuum oven, the sponge was cut into small ribbons with thickness of 1 mm and an area of 2 cm2. The sponge ribbons were subsequently coated using CNT ink suspension by a simple “dipping and drying” process. The next step was to electrodeposit MnO2-NPs on the CNT-coated sponge by galvanostatic “electrochemical deposition” for different times ranging from 3 to 40 min. Due to the mechanical flexibility of CNTs and strong VdWs interactions between the macroporous sponge cellulose and CNTs, the CNTs can be easily also coated onto the skeleton of a sponge, rendering the insulating sponge highly conductive by a simple “dipping and drying” process steps. Flower-like MnO2-NPs were uniformly deposited onto the conductive CNT sponge skeleton, even at the edges. This further confirms that CNTs have been conformably coated on the sponge. Field emission scanning electron microscope (FESEM) micrographs in Fig. 4(k) also shows an exciting point: the backbone of sponge is free of junctions and promotes the continuous coating of CNTs to form excellent conducting pathways in the whole structure. After deposition of MnO2, the highly porous nanostructure remained, which is good for faster transportation of electrons and ions in the SC devices.19,21
 | ||
| Fig. 4 Fabrication and surface morphology of porous textile conductors: (a) schematic of a 3D porous structure of cellulose fibers wrapped with CNTs, (b) conductive textiles fabricated by dipping into an aqueous CNT, (c) a thin, 10 cm × 10 cm textile conductor based on a 100% cotton fabric sheet, SEM image of (d) macroporous structure of SWCNTs coated cotton sheet, (e) fabric sheet coated with CNTs on the fiber surface and (f) high-magnification SEM image showing the conformal coating of CNT covering and bridging between the fabric fibers, (g) TEM image of CNTs on cotton fibers, (h) schematic drawing of electrodeposition of MnO2-NPs in the interior of porous structure of the SWCNT coated textile, SEM of (i) a top view of conductive textile after MnO2 coating, cotton fibers in the textile after peeling the fiber layers apart, high-magnification view image showing the flower structure of MnO2-NPs on SWCNTs. Reprinted with permission from ref. 3; (j) schematic illustration showing a fabrication process of hybrid MnO2/CNT/cotton sponge based SCs, (k) FESEM micrographs show an overall view of 3D macroporous hierarchical MnO2–CNT/sponge, MnO2 uniformly deposited on the skeleton of CNT/sponge, high magnification of porous MnO2 flower-like NPs on CNT/sponge. Reprinted with permission from ref. 21. | ||
The potentiometric chemical sensing cotton yarns have been coated using CNTs, and then partially covering them with the ion selective polymeric membrane. The potassium ion-selective membrane was a mixture of 2 wt% (18 mmol kg−1) of valinomycin, 0.5 wt% (10 mmol kg−1) of potassium tetrakis (4-chlorophenyl) borate, 32.8 wt% of poly(vinyl chloride) (PVC) and 64.7 wt% of bis(2-ethylhexyl) sebacate. To turn the cotton threads into conductive yarns successive “dipping–dyeing” or “dyeing–rinsing” steps can be applied (Fig. 5(a) and b). In each step, the cotton yarn was completely immersed in the CNT-ink (3 mg mL−1 in water with 10 mg mL−1 SDBS) for a few seconds. The cotton yarns, which immediately acquired the characteristic black color, were then removed by rinsing thoroughly with distilled water to eliminate the excess of surfactant. Interestingly, during the “rinsing” step, there was no visual evidence of the elimination of the CNT (which remain strongly adsorbed onto the yarn) as the rinsing water emerges clean (only revealing the presence of the surfactant). After rinsing, the cotton yarns were air-dried. These “dyeing–rinsing” steps can be repeated until a suitable value of electrical resistance is achieved. A whole sequence of the steps required to build the conductive CNT yarn based electrodes using the CNT coated cotton is shown in Fig. 5(c–e). As a first approach, a pipette tip (Fig. 5(c)) was used, in order to leave exposed only a fraction of the yarn, while the rest can be protected and can be accessed through the back-end of the tip. The exposed fraction of about 5 mm was fully dipped for a few seconds into a cocktail containing the ion selective membrane components (Fig. 5(d)), was then removed and dried for a few minutes. The number of times the dip coating can be performed to achieve optimum response. The final prototype of the yarn electrode (Fig. 5(e)) has the sensing end, and a back-end that can be connected to the reading instrument.6 The CNT ink can stay well disperse (Fig. 5(f)) for more than 30 days, so it can be applied on the surface of the cotton yarn by “soaking” step as shown in Fig. 5(g). However, various coating methods such as “drop casting”, “brushing” and “inkjet printing” can also be used. A particular challenge in e-textile demonstrations has been the process-induced strain as the yarn suffers from bending stress during the weaving process. As shown in Fig. 5(h), the inks made with metallic CNTs (5 mg mL−1 in water with 10 mg mL−1 SDBS) were first coated on both sides of the yarn and then the sensing CNT ink was “drop-coated” at the centre of the conducting CNT. Fig. 5(i) shows the images of cotton yarn coated with metallic CNT inks for electrodes and subsequently sensing CNT “drop-coated” at the centre. The control of the distance between CNT electrodes tends to be difficult as the process relies on dip coating.4
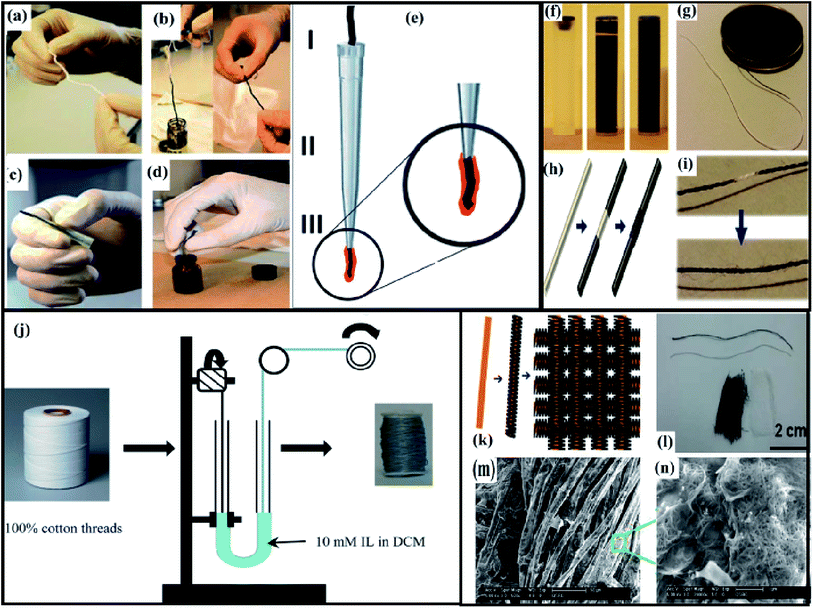 | ||
| Fig. 5 Detail construction of the CNT–cotton sensing electrodes: (a) Bare cotton yarn, (b) dyeing with CNT inks, (c) shielding with a pipette tip, and (d) dip coating into the membrane cocktail, (e) schematic of the prototype electrode: (I) connection of the CNT yarn to a measuring device, (II) pipette tip (shield), and (III) membrane coated CNT yarn end. Reproduced from ref. 6 with permission from The Royal Society of Chemistry; (f) CNT ink cartridges: empty, as-synthesized ink, and the ink after 1 month. Well dispersed CNT after 1 month, (g) cotton yarn dyed with CNT ink after immersion, (h) schematic illustration showing the fabrication of sensor based on the metallic CNT coated cotton yarn followed by drop-casting of the CNT at the center of cotton yarn, (i) photographic image of metallic CNT electrodes as a sensor. Reprinted with permission from ref. 4; (j) schematic representation showing the staining procedure of the cotton threads with 10 mM solution of ILs in DCM. Slow constant wounding of the coated cotton threads, yields an evenly stained spool. Reproduced from ref. 18 with permission from The Royal Society of Chemistry; (k) schematic illustration showing the fabrication procedure of preparing flexible CNT functionalized cotton fabrics, (l) photographs of bare cotton and CNT functionalized cotton fabrics, (m and n) SEM image of CNT functionalized cotton fabric. Reproduced from ref. 34 with permission from The Royal Society of Chemistry. | ||
Fig. 5(j) shows a schematic representation of “staining” the cotton threads using ionic liquid (IL). In order to stain the natural white cotton threads, 10 mM solution of IL was prepared by dissolving pure IL–methyl orange mixture in dichloromethane (DCM)–water by 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Then 10 mL of each solution was placed in a U-shaped tube and the cotton threads were passed through the tube and wound around a different spool. Afterwards, the IL-stained threads were sewn onto a cotton fabric template using a sewing machine.18 Fig. 5(k–n) shows the SEM micrographs and schematic illustration of the fabrication procedure involved in the functionalization of cotton fabrics using CNT ink (0.5 mg mL−1 in water with 10 mg mL−1 SDBS) followed by heating for 10 min at 120 °C.34
1 ratio. Then 10 mL of each solution was placed in a U-shaped tube and the cotton threads were passed through the tube and wound around a different spool. Afterwards, the IL-stained threads were sewn onto a cotton fabric template using a sewing machine.18 Fig. 5(k–n) shows the SEM micrographs and schematic illustration of the fabrication procedure involved in the functionalization of cotton fabrics using CNT ink (0.5 mg mL−1 in water with 10 mg mL−1 SDBS) followed by heating for 10 min at 120 °C.34
Stevan et al.116 coated CNTs on spider silk fiber by coating assisted by a water and mechanical shear method to produce tough, custom-shaped, flexible and electrically conducting fibers after drying and contraction. These conductive textiles were used to fabricate proof-of-concept sensor and actuator sensitive to strain and humidity.116 A neat bundle of multiple dragline 2 cm long fibers in their natural double-stranded arrangement (each strand has a diameter of 4 mm), turned very black, and when dried, contracted to a well-defined geometry, where the fibers were uniformly coated with a dry powder of functionalized CNTs, applying a few drops of water, and then pressing and shearing the mixture between two Teflon sheets (Fig. 6(a)). After the coating process, the dragline fibers were separated into individually coated single-strand fibers, accompanied by small, isolated CNT aggregates, which allowed reliable extraction of single fiber from the bundle. SEM and TEM images (Fig. 6(a)) of the single silk fiber show that the CNTs are attached to the fibrous structure, including some penetration of the nanotubes into the SS surface. This procedure produces a basic uniform annular CNT coating with a thickness of 80–100 nm with occasional CNT aggregates of 1 mm in diameter and thickness. Pre-supercontracted fibers were also coated by water-based procedure, first immersing the neat fibers in a water bath for 30 min, followed by air drying, and then the water-based CNT coating, indicating that the initial shrinkage of fibers is not the most important factor to achieve the effective coating, but it softens the fibers during supercontraction.116 In reported work by Zheng et al.117 Ni-coated cotton yarns were fabricated by a “polymer-assisted metal deposition” method (Fig. 6(b)). In a typical experiment, commercially available, pre-cleaned cotton yarns were dipped into an ethanoic solution of poly[2-(methacryloyloxy)ethyl trimethylammonium chloride-co-3-(trimethoxysilyl) propyl methacrylate] [P(METAC-co-MPTS)]. After hydrolysis and curing steps, 10 nm-thick P(METAC-co-MPTS) was covalently grafted onto the cotton surfaces. Subsequently, the copolymer-grafted cotton yarns were immersed into an aqueous solution of (NH4)2 and PdCl4, where PdCl4 were loaded onto the copolymer layer through the strong ionic interactions with the quaternary ammonium groups. Finally, the samples were immersed in an ELD bath of Ni for a certain time, in which a thin layer of Ni was deposited on the surface of the cotton yarn. This fabrication was highly scalable, because the process was performed in a solution manner. Fig. 6(b) also shows an as-made 500 m-long Ni-coated cotton yarn that was wound on a spinning cone. The average thickness of the Ni-coated cotton yarns was 0.45 mm. As chemicals were able to penetrate into the inner space of the cotton yarns during wet processing, Ni was uniformly and densely coated on the surfaces of both the outer and inner cotton fibres of the yarn (Fig. 6(b)). The thickness of the Ni coating increased from 260 to 650 nm, as the ELD time increased from 30 to 120 min. To fabricate the composite electrodes, graphene was deposited on the surface of Ni cotton yarns by chemical electrolysis using 3 mg mL−1 GO aqueous suspension at an applied potential of 1.2 V for a certain time, in which the Ni cotton yarns were used as working electrodes. It was observed that rGO flakes penetrated into the multiple interval space among the individual fibers of the Ni cotton yarns. With an increasing electrochemical deposition time, the rGO coating became thicker and denser (Fig. 6(b)). It was observed that the Ni coated cotton yarn was fully covered with rGO flakes after 20 min electrochemical deposition.117
 | ||
| Fig. 6 (a) SEM image of CNT-SS with a diameter of 6.5 mm (scale bar: 10 mm), magnified SEM images of CNT-SS surface showing a uniform mat-like covering (scale bars: 1 mm), TEM cross section of a dragline silk fiber with CNT coating (scale bar: 1 mm), TEM image indicating nanotube penetration (red arrows) into the silky structure (scale bar: 250 nm). Reprinted with permission from ref. 116; (b) schematic illustration of the fabrication of rGO/Ni cotton yarn composite electrodes, digital image of a 500 m long Ni-coated cotton yarn wound on a spinning cone, high-magnification cross-sectional SEM micrograph of a Ni-coated cotton yarn, SEM micrographs of a typical rGO/Ni cotton composite electrode made with 10 min rGO electrochemical deposition. Reprinted with permission from ref. 117. | ||
Antibacterial textiles can be prepared by coating the ZnO-NPs onto cotton fabrics to enhance UV-blocking, self-cleaning and antibacterial properties. In order to produce durable antibacterial textiles, various pre- or post-treatments, such as coating with water insoluble polymers, use of cross linking agents or plasma treatments have been reported to improve the stability of the deposited antimicrobial NPs on cotton fabrics. The cotton fibers were pre-treated in order to remove various non-cellulose components, such as wax, grease, and other finishing chemicals. Bleached cotton woven textile samples were washed in water bath at 60 °C for 3 h after the samples were washed in a washing machine without using detergent. The samples were then washed three times with cold DI water, dried in oven at 60 °C overnight and cut (3 cm × 3 cm) for UV-blocking and antibacterial assays, respectively. The samples were stirred for 30 min in solutions of ZnO-NPs and the wet samples were left for 10 min to enable solvent evaporation at room temperature and then were put in a vacuum oven for 5 min at 135 °C for further binding. A schematic representation of possible interaction events between ZnO-NPs and cotton fabrics are shown in schematic of Fig. 7(a). Bare cotton fiber showed a smooth surface texture as shown in Fig. 7(b–d), whereas ZnO-NPs treated samples showed that cotton fibers were covered with a uniform and dense distribution of ZnO-NPs as shown in Fig. 7(e–g). Fig. 7(g) showed the aggregation of these NPs and the heterogeneous presence of ZnO-NPs over the cotton surface. The EDS result confirmed that a lot of ZnO nanocrystallites were deposited all over the inner structure of cotton fibers, and this was in agreement with the FESEM results as shown in Fig. 7(c).45 Gogosti et al.106 used various methods (dip coating, screen printing and electrochemical deposition) for carbon deposition on cotton based textile materials. He reported that dip coating may not be capable of penetrating and coating of carbons through such a thick and dense material. No uniform coating on the textile was achieved and the resulting coatings were not dense enough to create conductive bonds similar to those in conventional thin film SCs. Screen printing provided full penetration of carbon through the yarns and into the fiber bundles as shown in Fig. 7(h–k). The cotton fabric alone weighs 6.8 mg cm−2 per electrode. However, both fabrics, regardless of mass or carbon uptake ability was impregnated with the same amount of carbon, on average 4.9 mg cm−2. Cotton lawn holds 81% of its weight carbon.106 Fig. 7(h and i) shows the thorough coating and adhesion of the carbon to the cotton fibers. Carbon distribution within the cotton textiles could change the electrical and electrochemical performance. The carbon network within the cotton lawn may have better continuity due to the highly porous curvilinear structure of cotton lawn fibers as shown in Fig. 7(j and k). The mass of the cotton lawn is also half, resulting in electrodes that contain more carbon and less substrate material.106
 | ||
| Fig. 7 (a) Schematics of interaction events between ZnO-NPs and cotton fabrics, FESEM images of surface morphology of: (b) pristine cotton fibers (MAGX70), (c) a higher magnification of (image b) (MAGX500); (d) a higher magnification of (image c) (MAGX5000), (e) cotton fibers coated with ZnO-NPs (MAGX90), (f) a higher magnification of (image e) (MAGX400), (g) a higher magnification of (image f) (MAGX5000). Reprinted with permission from ref. 45; SEM images of (h) cotton lawn plain weave before coating, (i) cotton fiber screen printed with carbon particles, (j) model of carbon impregnation into CFs organically shaped 16–30 mm width structure allows for improved impregnation of carbon particles and ion transport, (k) structural formula of cotton. | ||
Sainy et al.97 prepared PPy coated conducting cotton fabrics (microwave absorbing material) by in situ chemical oxidative polymerization using an oxidant. The cotton fabric (15 cm × 15 cm) was dipped in 200 mL aqueous solution of 0.1 M pyrrole in a glass trough. An aqueous solution of 0.2 M FeCl3 was added drop wise over a period of 2 h and as a result polymerization was initiated at 23 °C with uninterrupted agitation throughout the course of the reaction. After, completion of polymerization, the PPy grafted fabric (cotton–PPy) was removed from the trough, thoroughly rinsed with distilled water and chloroform and dried at 60 °C under vacuum.97,115 CP like PEDOT:PSS (Poly (sodium styrene sulfonate)) have been can be reinforced on the surface of cotton by simple “soaking” process to prepare flexible conductive threads which can be used as a channel of an OECT, directly interfaced with a liquid electrolyte in contact with an Ag-wire gate. The cotton–OECT channel by simply soaking cotton yarns into an aqueous solution of p-type conductive PEDOT:PSS for 5 min and by baking on a hot plate at 150 °C for 2 h. The PEDOT:PSS solution can be modified by adding 20% ethylene glycol and 5% dodecyl benzene sulfonic acid (DBSA) surfactant to increase electrical conductivity and to decrease solubility in water. Conductive thread turned out to become a wire with an electrical resistance of 430 Ω cm−1. After processing with PEDOT:PSS, the threads (100–110 S cm−1) still maintain their flexibility and can be easily integrated on cloth.16,20,32,115 Zou et al.37 used dipping–coating method to coat flexible textile threads using same CP (PEDOT:PSS) doped with Di methyl sulfo-oxide (DMSO). The dipping–coating times can be varied to easily adjust the PEDOT:PSS loading on the thread. The PEDOT:PSS loading was found to increase linearly with dipping–coating times, whereas the resistance of the thread linearly decreased to 13 Ω cm−1 (i.e. conductivity of 109 S cm−1). The fabrication of the highly conductive threads is an important step in the flexible textile based electronics field. Therefore, the conductive thread could be bended or knotted, while the conductivity and mechanical properties sacrificed a little, which are the basic requirements for weaving the thread into electronics.37
Electronic functions can be integrated in cotton fabric using “surface mounting”, conductive “nanocoating” and “self-organizing” technologies. “Surface mounting” technology used in electronic industry is related to “lamination” technology of the textile industry. The thin-film based devices can be attached onto conventional textile fabrics by thermoplastic adhesives. Apart from that, fabrication of free standing electronic devices directly on textile substrates can be achieved by three technologies, i.e. “screen printing”, “digital printing” and “dip-coating”, which have been developed to fabricate a processable solution for wearable devices in textile substrate. One key advantage of these methods is that they facilitate the use of low-cost patterning techniques at room condition. “Screen printing” screen provides an easily adopted fabrication route for the fabrication of wearable electronics, all the layers with different functions are printed on top of the fabric substrate through a label process. This process does not need extra photolithography and chemical etching processes as each structural pattern is directly defined with every layer application. In addition, “screen printing” is also compatible with industrial roll-to-roll processes, offering a route to high volume batch fabrication, e.g. fabric strain sensor screen-printed with activated carbon and textile energy storage devices made by “screen printing” activated carbon paint onto custom knitted fabrics. Further, the “screen printing” method has excellent applicability on any irregular textile surface that can offer significantly more design freedom and placement capability on fabrics than other methods like weaving and knitting. Compared to the “screen printing” technology, “digital printing” technology has the advantage of high spatial precision of ink droplet. Combined with “inkjet printing” provides an exciting opportunity to apply on-demand material deposition and desktop programmable wiring of designed patterns. The latter has already been demonstrated for metal, CNT and graphene based inks. In addition, piezoelectric, piezoresistive and capacitive elements also can be developed by “digital printing” technology for detecting deformation of a fabric. Conductive “nanocoating” technologies are another effective approach to integrate electronic functions within fabrics and improve the performance and functionality of wearable electronics. Self organizing technology is also an important technique to obtain conductive fabrics, e.g. a fiber-based micro-SC uses piezoelectric ZnO-NWs grown self-organized and radially around the fibers.11,12
3.2 Characteristic properties
Fig. 8(a–d) shows series of various morphologies of e-textile indicating that both SWCNTs and MWCNTs stabilized in Nafion (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of CNTs/Nafion) seamlessly cover the exterior of every strand of cotton yarn in such a way that numerous electrical paths can be formed. SWCNTs form a tighter and more dispersed network than large and rigid MWCNTs, easily recognizable even at low magnifications (Fig. 8(d)) because the uniformity of nanotube distribution strongly affects both strength and conductivity of CNT composites. These conductive cotton yarns and fabrics have been prepared by Shim et al.1,2 for human biomonitoring and telemedicine sensors such as humidity sensing, anitigen/antibody sensing for albumin biosensor.1,2 As shown in Fig. 8(e), a two-meter-long SWCNTs coated cotton thread can be easily wound on a Teflon rod. In addition, Fig. 8(f) illustrates the same cotton thread being stretched. This means that the highly conductive fiber can still retain its flexibility and foldability after uniform coating of SWCNTs. Fig. 8(g) shows SEM which reveals the microstructure of a conductive cotton thread. Uniform coating of SWCNT cross-linked networks is also observed in Fig. 8(h). From the cross-sectional SEM images shown in Fig. 8(i and j), it can be seen that a well-bundled SWCNT film with thickness of about 800 nm densely coats the entire micro fibril. This kind of nanostructured conductive cotton threads has been reported in the development of cable-type SCs for wearable energy storage devices.5
1 weight ratio of CNTs/Nafion) seamlessly cover the exterior of every strand of cotton yarn in such a way that numerous electrical paths can be formed. SWCNTs form a tighter and more dispersed network than large and rigid MWCNTs, easily recognizable even at low magnifications (Fig. 8(d)) because the uniformity of nanotube distribution strongly affects both strength and conductivity of CNT composites. These conductive cotton yarns and fabrics have been prepared by Shim et al.1,2 for human biomonitoring and telemedicine sensors such as humidity sensing, anitigen/antibody sensing for albumin biosensor.1,2 As shown in Fig. 8(e), a two-meter-long SWCNTs coated cotton thread can be easily wound on a Teflon rod. In addition, Fig. 8(f) illustrates the same cotton thread being stretched. This means that the highly conductive fiber can still retain its flexibility and foldability after uniform coating of SWCNTs. Fig. 8(g) shows SEM which reveals the microstructure of a conductive cotton thread. Uniform coating of SWCNT cross-linked networks is also observed in Fig. 8(h). From the cross-sectional SEM images shown in Fig. 8(i and j), it can be seen that a well-bundled SWCNT film with thickness of about 800 nm densely coats the entire micro fibril. This kind of nanostructured conductive cotton threads has been reported in the development of cable-type SCs for wearable energy storage devices.5
 | ||
| Fig. 8 SEM images of e-textiles: (a and b) SWCNT-Nafion coated and (c and d) MWCNT-Nafion coated cotton threads after one dipping cycle. Reprinted with permission from ref. 1; Optical images of a two-meter-long SWCNTs coated cotton thread, (e) wound on a Teflon rod, and (f) the same thread in a stretched state, (g) SEM image of the SWCNTs coated cotton thread revealing its macro porous structure, (h) High-magnification SEM image showing the uniform coating of SWCNT cross-linked networks, (i and j) cross-sectional SEM images of a SWCNTs coated micro fibril. Reprinted with permission from ref. 5; cut view of the membrane: (k) view of the CNT coated yarn, (l) zoomed view of a portion of the membrane. Reproduced from ref. 6 with permission from The Royal Society of Chemistry; SEM images of Au-NW-coated (m and n) cotton thread. Reproduced from ref. 33 with permission from The Royal Society of Chemistry. | ||
After the dip-coating (4–5 dip-coating cycles) of the CNT on cotton yarn into the ion-selective membrane cocktail, a smooth membrane with optimum performance that covers the whole exposed tip can be obtained. A cut view of SEM images of this membrane can be seen in Fig. 8(k and l) and an estimated thickness of the membrane of approximately 100 microns was found. It should be noted that the membrane reaches the plastic of the pipette tip, preventing any direct contact of the solution with the CNT–cotton yarn. The clean bare cotton yarns contrasts with the dense network of CNTs adsorbed on the surface. This is a very attractive feature of these systems, a conductive 3D network wrapped around each cellulose fiber is clearly seen. These kind of morphology are suitable for potentiometric sensors, including pH, K+ and NH4+ sensors.6 Fig. 8(m and n) shows SEM images of Au-NWs coated cotton threads. The cotton thread was dipped 3 times in a NW solution with a concentration of 5 mg mL−1 and has a resistance of 11 Ω cm−1. In addition, other textile material such as the nylon and polyester threads were also studied. These all conductive textile threads have been demonstrated as heaters.33
The PEDOT:PSS treatment improved the appearance of the commercial conductive threads, as shown in Fig. 9(a and b), which are high-oriented monofilaments and can be twisted together. The monofilaments were adhered closely together by PEDOT:PSS and formed a composite threads of approximately 300 mm diameter. The surface of the thread was well covered with the conductive PEDOT:PSS film and the parallel inlet on the film caused by the monofilaments under surface was easily observed from cross-section SEM images as shown in Fig. 9(c and d). Moreover, the PEDOT:PSS film surrounding the cotton threads was in the nanometer range. These flexible cotton threads have demonstrated as wearable dye-sensitized solar cells.37 Fig. 9(e) shows a FESEM image of a thin layer of PEDOT:PSS surrounded cotton yarn. The layer appears uniformly distributed in the nanometer scale with an estimated thickness of about 50 nm; few layer borders could be seen on the side of the yarn. After processing with PEDOT:PSS, the yarn still maintained its flexibility and can be easily integrated on cloth without altering output characteristics. This property is very important and useful for real e-textile applications, e.g. biosensor for monitoring of human stress, integration of the device in the fitness or daily routines' shirts.32 Gao et al.5 observed SEM images of flower-like MnO2 nanostructures homogeneously grown on the conductive cotton threads, which clearly show a 3D hierarchical structure as shown in Fig. 9(f and g). The surface of the conductive cotton fiber can be fully covered by highly porous, crystalline and tiny nanoplates of MnO2 structures after 20 min of “electrochemical deposition”. But some cracks appear on the MnO2 nanostructures after 60 min, probably due to the too thick structures. These metal oxide coated cotton threads were prepared for cable-type SC application. As shown in Fig. 9(h), one can observe that PPy film is uniformly deposited on the whole MnO2–CNT–cotton thread structure, ranging par from the top of MnO2 nanostructures to the SWCNTs at the bottom, without changing the overall porous morphology. The thickness of the PPy wrapped MnO2 composites on cotton threads was about 2–3 μm. This ultrathin layer of PPy film wrapped around the flower-like MnO2 nanostructures provides an additional electron transport path and actively participates in the energy storage process, which improves the SC performance.5 Liu et al.30 reviewed various flexible SCs by using carbon cloth current collectors to support NWs, e.g. asymmetric SCs based on acicular Co9S8–NR arrays as positive materials and Co3O4–RuO2 nanosheet arrays as negative materials as shown in Fig. 9(i and j). The fabric electrodes can be prepared by dipping the non-woven cloth into a dispersion of CNTs and subsequent MnO2 electrodeposition to construct individual electrodes with lamination configuration, that enable fold-increased areal capacitances and excellent cycling stability. While in the tandem configuration, each unit SC was made of two pieces of MnO2/CNT electrodes sandwiched with H3PO4/PVA solid-state electrolyte with a high-output-voltage device.24,30,104
 | ||
| Fig. 9 Morphology of the PEDOT:PSS coated conductive thread: (a and b) SEM images of the conductive thread and (c and d) cross-section SEM images of the conductive thread. Reproduced from ref. 37 with permission from The Royal Society of Chemistry; (e) FE-SEM image of the cotton yarn/wire functionalized with PEDOT:PSS, treats are uniformly covered, the PEDOT:PSS film thickness is randomly highlighted by some fringes at the treat borders. Reproduced from ref. 32 with permission from The Royal Society of Chemistry; SEM micrographs of (f and g) flower-like MnO2 nanostructures grown on the whole conductive cotton thread, 3D thread multi-grade nanostructures (MnO2–CNT–cotton) with (h) PPy deposited over 1.5 min. Reprinted with permission from ref. 5; SEM images of (i and j) Co3O4 and Co9S8 acicular NR arrays grown on carbon cloth. Reprinted with permission from ref. 104. | ||
 | ||
| Fig. 10 (a) SWCNTs coated cotton thread during passing the tape test, indicating strong adhesion and mechanical rigidity. Reprinted with permission from ref. 5; (b) stress–strain curves for the CNT–cotton yarn and the original cotton thread. Reprinted with permission from ref. 1; (c) sheet resistance of fabric and cotton sheet after SWCNT coating. Reprinted with permission from ref. 3; (d) the relationship between line resistance and mass loading of SWCNT. Reprinted with permission from ref. 5; (e) resistance as a function of the number of dipping cycles, (f) (I–V) measurements of CNT functionalized cotton fabrics with short (DS) and long (DL) lengths. Reproduced from ref. 34 with permission from The Royal Society of Chemistry; (g) output (I–V) characteristics of the cotton–OECT based on PEDOT:PSS realized with 0.1 M NaCl. Reproduced from ref. 32 with permission from The Royal Society of Chemistry. | ||
Mechanical (tensile) tests can also be carried out by gripping the two ends of CNT entangled yarn and stretching the entanglement into a predefined strain.44 Gao et al.73 synthesized wearable and shapeable GFs and reported their tensile strength up to 180 MPa and an elongation at break of 3–6%. The strong and flexible hollow GFs are mechanically stable, flexible and can be woven into engineered structures. Notably, these GFs are flexible; can be fastened into tight knots without any breakage, or integrated into conductive patterned textiles with other threads.20,73 Cheng et al.27 reviewed and reported mechanical flexibility of LIBs prepared from nanostructured (activated carbon fabric (ACF)/TiO2 nanosheets) electrode materials due to its large surface area, high electrolyte adsorption capability and excellent mechanical flexibility. Combining the high pseudo capacitive TiO2 with a strong ACF, the self-supporting TiO2/ACF film electrode with high tensile strength (12.7 MPa) is promising for flexible LIBs.27 Lozano et al.74 also observed mechanical properties of pristine nylon-NFs (of 34 MPa) and CNT–nylon NFs, and found 338% enhancement in tensile strength as compared to that of the pure nylon NFs, whereas the strain at break was reported to be decreased from 417 to 250% with the incorporation of 1 wt% pristine SWCNT in nylon–NFs.74
 | ||
| Fig. 11 (a) Schematic diagram of the CNT functionalized cotton heaters, (b and c) Heating experiments of CNT functionalized cotton fabrics, temperature as a function of time for long (DL) and short (DS) length, the inset shows the optical image of the heaters. Reproduced from ref. 34 with permission from The Royal Society of Chemistry; (d) temperature profiles of the thread heater at different input voltages, voltages were applied across a 1.4 cm long section of thread for 150 s. Reproduced from ref. 33 with permission from The Royal Society of Chemistry. | ||
| Name of linear and flexible nanostructured material for wearable devices | Specific capacitance, F g−1 or | Areal capacitance, mF cm−2 | Power density, kW kg−1 | Energy density, W h kg−1 | Application |
|---|---|---|---|---|---|
| SWCNT/MnO2 coated cotton threads and fabric3,26,30 (conductivity of 5–125 S cm−1 and sheet resistance 4 Ω cm−2 and high specific energy)3 | 140 at 20 μA cm−2 and 80 at 20 mA cm−2 | 480 | 10 | 20 | Lightweight, flexible, stretchable, porous, and conductive energy textiles for SC application3 |
| Cotton/MnO2/SWCNT/PPy,5 cotton/MnO2/SWCNT3,30 | — | 410,3 149, 520![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 at 1 mV s−1 5 at 1 mV s−1 |
0.67–13.29 mW cm−2 | 14.7–33 μW h cm−2 | 3D cable type, flexible, light weight, foldable, wearable, energy storage |
| Cotton–rGO@CMC,7 cotton–CNT@CMC,7 cotton–rGO–CNT@CMC,7,20 ultra-high flexibility with elongation of 8–10% and mechanical strength (73–116 MPa) | — | 127, 47, 177, 269 | — | 3.84, 5.91 mW h cm−2 | Two-ply yarn SCs7 |
| MnO2/sponge,19,21 MnO2/CNT/sponge19,21 | 1400, 1230 | 520 | 63 | 31 | 3D supercapacitor19 with safety |
| Cotton/carbon sphere or GO sheets23 | — | 53.56 mF cm−2 | — | 7.96 × 10−5 W h cm−2 | FFSCs19 |
| Cotton cloth/CNTs/RuO2-NWs26 | 138 | — | 96 | 18.8 W h kg−1 | SCs26 |
| Cotton sheets/SWCNTs26 | 70–80 | — | — | — | — |
| Carbonized cotton mats26 | 12–14 | — | — | — | — |
Activated cotton T-shirt textile/MnO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
269.5 | — | 4.97 | 66.7 | — |
| ZnCo2O4-NWs array/carbon cloth27 | 1200–1340 mA h g−1 | — | — | — | LIBs27 high flexibility, superior rate capacity and lithium storage capability |
| Cotton/CNT@PAni@PVA25,30 | — | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
— | — | Two ply yarn SCs25 |
| CNTs/cotton lawn or twill,106 cotton/porous carbon26 | 85–95 at 0.25 A g−1 | 430 F cm−2 at 5 mA cm−2 | — | — | Smart garments for flexible energy storage106 |
Gao et al.7 studied the electrochemical properties of two-ply YSCs (neat rGO@CMC and neat CNT@CMC assembled coaxial fibers) characterized by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) measurements. For both types of YSCs intertwined with rGO@CMC and CNT@CMC fibers (knitted in cotton materials), the CV curves showed a nearly rectangular shape and a rapid current response to voltage reversal at each end potential, which illustrates the good electrochemical performance of the YSCs. The GCD behavior of rGO@CMC YSCs characterized at different current densities (0.1–1.0 mA cm−2) between 0 and 0.8 V showed triangular shape, confirming the formation of an efficient electric double layers and good charge propagation across the two fiber electrodes. Areal capacitance (CA), length capacitance (CL) and volume capacitance (CV) are commonly utilized to evaluate the charge-storage capacity of YSCs, which were reported extremely high (CA = 127 mF cm−2, CV = 114 F cm−3, CL = 3.8 mF cm−1 for rGO@CMC based YSCs and CA = 47 mF cm−2, CV = 42 F cm−3, CL = 1.4 mF cm−1 for CNT@CMC) at the current density of 0.1 mA cm−2. The capacitance of SCs at high charge–discharge current density is also crucial for their practical application as wearable energy-storage devices.7 Zhu et al.22 reported the total length specific capacitance of electrochemically reduced graphene oxide (ERGO)@CF-H/PVA-H3PO4 based SC reported to be up to 13.5 mF cm−1 at a current density of 0.05 mA cm−2. Energy density and power density are two key parameters to determine the quality of a capacitor, which were reported to be 1.9 mW h cm−1 at a power density of 27.2 mW cm−1. Such a charged SC is capable of lighting up a red LED.22 Lin et al.23 studied the electrochemical performance of GO nanosheet and carbon nanospheres hierarchical nanostructure (GCHN) electrodes and the corresponding optimal fibrous, flexible supercapacitor (FFSC) devices (50 wt% GO content) characterized by CV, GCD, and electrochemical impedance spectroscopy. The as-prepared GCHN, FFSC electrode served as the working electrode; a Pt wire and a saturated calomel electrode (SCE) were used as counter and reference electrodes, respectively. All CV, GCD curves were linear and symmetric and close to a triangular shape, signifying the typical electrical double-layer capacitors (EDLC) behavior. Clearly, the shapes of the electrodes demonstrated the excellent electrochemical reversibility and charge/discharge properties.23 The catalytic performance of the PEDOT:PSS coated conductive cotton threads have been investigated from CV curves. The CV curves of conductive threads with different PEDOT:PSS loadings showed the high catalytic performance ascribing to PEDOT modification. The electrochemical properties showed the potential application of conductive threads in DSSCs.37
Shen et al.30 reviewed and reported about low-cost, flexible, stretchable and lightweight cotton cloth to be ideal substrates for wearable SCs. Cui et al.3 tested porous textile conductor with coated with SWCNT as both active charge storage electrodes and current collectors in SCs. The uniformly coated SWCNTs make these textiles highly conductive with sheet resistance less than 4 Ω cm−2. The linear voltage–time profile (Fig. 12(a)) confirms the charging and discharging of the SCs. The specific capacitance of SC with porous textiles was around 2–3 times better than that with polyethylene terephthalate (PET) substrates in the range of current density 20 μA cm−2 to 20 mA cm−2 (Fig. 12(b)). The SCs made from these conductive textiles with large CNT loading mass (up to 8 mg cm−2) showed high CA (Fig. 12(c)), up to 0.48 F cm−2 and good cycling stability. In the Ragone plot (Fig. 12(d)) The masses of the electrode materials (16 mg cm−2), cotton cloth (24 mg cm−2), electrolyte (6 mg cm−2), and separator (2 mg cm−2) were included in the complete SC device, which achieves a high energy density of 20 W h kg−1 at specific power of 10 kW kg−1. When substituting the cotton cloth with stretchable fabrics, a flexible and stretchable SC is also feasible, showing excellent stability even after thousands of cycles (Fig. 12(e)). Fig. 12(f) indicates the specific capacitance of SC before and after being stretched up to 120% strain 100 times. To demonstrate the feasibility of this pseudo-capacitor approach for wearable power devices, SCs of SWCNT/cotton with MnO2 were also tested with a 2 M aqueous Li2SO4 electrolyte. The time required to charge the SCs for SWCNT/cotton after MnO2 deposition was significantly increased, suggesting a large charge capacity increase, which can be confirmed from Fig. 12(g). The CA with respect to the device was increased by a factor of 24 after MnO2 deposition. The CA of the device was reaching up to 0.41 F cm−2, which was much higher than values with SWCNT electrodes. The specific capacitance data, considering the mass of both SWCNTs and MnO2 is plotted in Fig. 12(h). The specific capacitance increased by a factor of 4 when including the masses of both CNT and MnO2. But it was noted that the specific capacitance of SWCNTs with Li2SO4 electrolyte was lower due to better wetting between the organic electrolyte and SWCNTs. Such wearable SCs with salt electrolyte show an excellent cycling stability (Fig. 12(i)) with negligible change between the initial and the final specific capacitance over 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.3,30
000 cycles.3,30
 | ||
| Fig. 12 Organic SC with porous textile conductor: (a) the linear voltage–time profile, (b) SC performance comparison between SWCNTs on PET and SWCNTs on cotton, (c) areal capacitance increases with the areal mass loading of SWCNTs, (d) Ragone plot of commercial SCs, SWCNT based SC on metal substrates, and SWCNT based SC on porous conductors including all the weight, (e) cycling stability of a SC with the porous textile conductor, (f) the specific capacity for a stretchable SC before and after stretching to 120% strain for 100 cycles at a current density of 1 mA cm−2, (g) charge–discharge of aqueous SC with SWCNT/cotton electrodes and 2 M Li2SO4 as the electrolyte with current of 20 μA cm−2, (h) the specific capacitance of SWCNT/cotton with and without MnO2 for different discharge current densities, (i) cycling stability of a SC with SWCNT–MnO2-NPs and porous textile conductor. Reprinted with permission from ref. 3. | ||
Porous and functionalized cotton threads can be used as both current collectors and active charge storage electrodes in SC testing. From Fig. 13(a and b), CV curves showed almost keep the rectangular shape (a feature of an ideal EDLC) even at a high scan rate of 2 V s−1. Fig. 13(c) illustrates the charge–discharge behavior of the SWCNTs coated cotton thread devices at a current density of 1.66 mA cm−2. An ultrafast charge–discharge rate, linear dependence of voltage on time, and a very small voltage drop are evident, indicating excellent SC performance. The SC performance was improved significantly after deposition of MnO2 nanostructure. Using a 45 min MnO2 deposited sample, the CV curves were recorded with having an almost rectangular shape below scan rates of 0.5 V s−1 as seen from Fig. 13(d). Moreover, Fig. 13(e) shows the CV curves of the conductive cotton produced at different deposition times at a scan rate of 100 mV s−1. A maximal CA of about 0.52 F cm−2 was reported for the conductive cotton with an optimized deposition time (45 min) at a scan rate of 1 mV s−1. At optimized deposition time, an optimum loading of MnO2 was used as a platform for the final PPy film deposition. Furthermore, PPy coated cotton thread sample yielded the highest areal capacitance with a value of 1.49 F cm−2 at a scan rate of 1 mV s−1. SWCNT backbone on cotton thread, active mesoporous flower-like MnO2 nanoplates, and PPy conductive wrapping layer can improve the electrical conductivity and acts as a pseudo capacitance material simultaneously. The SC based on the PPy–MnO2–CNT system also yielded a higher areal energy density of 33 W h cm−2 at a power density of 0.67 mW cm−2 and a high areal power density of 13 mW cm−2 at an energy density of 14.7 W h cm−2. These values demonstrate that 3D (PPy/CNT/MnO2) nanostructured cotton thread based electrodes are promising candidates for application in cable type SC.5 In order to test electrochemical performance of the wearable textile (Ni coated textile threads) battery consisting of the Li4Ti5O12 (LTO) anode and the LiFePO4 (LFP) cathode under severe mechanical motions, Choi, Lee and Kim et al.13 used a home-built folding instrument for in situ battery measurements during repeated folding–unfolding.13
 | ||
| Fig. 13 CV curves of a SWCNTs coated cotton thread based device with scan rate (a) from 0.001 to 1 V s−1 and (b) from 1 to 5 V s−1, (c) GCD behavior of the device at a current density of 1.66 mA cm−2, CV curves of the device (d) with MnO2 deposited over 45 min at a scan rate from 0.001 to 0.2 V s−1, and (e) with different MnO2 deposition times at a specific scan rate of 100 mV s−1. Reprinted with permission from ref. 5. | ||
4 Flexible smart devices based on hybrid nanostructured conductive cotton materials
Wearable devices have revealed numerous brilliant and smart designs with applications, showing valuable gimmicks like adaptability, light weight and foldability. With the end goal being able to attain wearable devices and smart textile, in the field of energy management and sensing displays and presentations, (e.g. sensing gadgets, convenient cum portable power gadgets and embedded vital signs monitoring devices) so forth a light weight device is essential. The CNMs, NMs, NPs hybridized flexible textile structure base electrodes are light, durable, foldable and comfortable thus ideal for the development of wearable devices.1–8,11,13,22,29,32 Table 3 shows various schematics of wearable and smart devices based on flexible and linear cotton materials which can be knitted with textile materials along with their applications in different fields.| Sr. No. | Schematic view | Application |
|---|---|---|
| I | 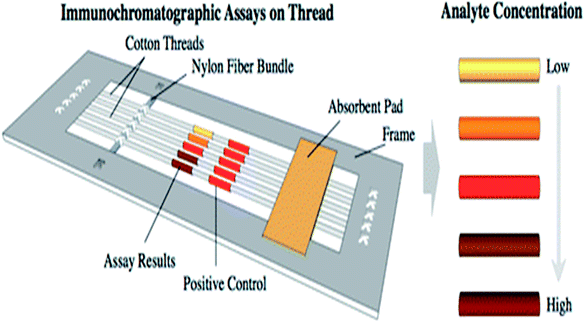 |
Au-NP based immunechromatographic assays developed on cotton thread. Reprinted with permission from ref. 122 |
| II |  |
Smart electronic yarns and wearable fabrics for human bio-monitoring made by CNT coated with polyelectrolytes (digital images of electronic circuits. Conductive yarn or fabric can also used as electrical wire or substrate for powering an LED). Reprinted with permission from ref. 1 |
| III |  |
(a) Cotton–OECT, directly integrated on cloth. On the top, OECT device is shown with an Ag-gate, while at the bottom the same device is shown with a Pt-gate. A drop of liquid electrolyte is placed in contact with the thread and the gate, the overlapping between the liquid electrolyte and the PEDOT:PSS wire defines the OECT channel, (b) schematic of the cotton–OECT device with a Pt gate and an adrenaline molecule in its sensing process. Reproduced from ref. 32 with permission from The Royal Society of Chemistry |
| IV |  |
The left image shows a schematic illustration of e-textile knitted with CNT–cotton yarn, right image shows an ammonia sensor on CNT–cotton yarn knitted ordinary textile. Reprinted with permission from ref. 4 |
| V |  |
(a) Illustration of a K+ sensing band-aid with the membrane-coated sensing section (A), the heat-shrink tape (B) and the connection to the reading instrument (C); (b) illustration of the placement of the sensing band-aid on a human model. Reproduced from ref. 6 with permission from The Royal Society of Chemistry |
| VI |  |
(A) Photonic IL sensor arrays fabricated on (i) silica, (ii) alumina and (iii) filter paper and their respective; (B) digital images, (C) cotton thread spools stained with chemosensory ILs (P refers to the ion), (D) sensor array fabricated from IL-stained threads: (i) by using a sewing machine and a (ii) hand-stitched ‘warner research’ logo. Reproduced from ref. 18 with permission from The Royal Society of Chemistry |
| VII |  |
Wireless body temperature sensor system triggered by the “power shirt”: (a) schematic diagram and (b) digital photography of a wireless body temperature monitor system. Reprinted with permission from ref. 8 |
| VIII |  |
Upper image shows picture illustrates the integration of the fiber into the textile to form the sensing setup, lower image shows the sensing system placed on a human subject study, blue and black connectors are housings for the LED and the photodiode, respectively, the power supply is provided by the grey wires. Reprinted with permission from ref. 118 |
| IX |  |
The left image shows the SC structure with porous textile conductors as electrodes and current collectors, the porous structure facilitates the accessibility of electrolyte, right image shows the schematic drawing of the stretchable SCs with SWCNT/fabric as electrodes and with stretchable fabric as the separator (top), a SC (bottom) under 120% strain condition. Reprinted with permission from ref. 3 |
| X |  |
Schematic diagram of a cable-type SC (the inset is a photograph of a twisting cable-type SC). Reprinted with permission from ref. 5 |
| XI |  |
(a) Two intact coaxial fibers woven with cotton fibers, (b) optical macroscopic image, (c) cloth woven by two individual coaxial fibers, (d) SC device based on the cloth fabricated by two coaxial fibers (denoted as i and ii, respectively). Reprinted with permission from ref. 7 |
| XII |  |
Schematic of a porous textile SC integrated into a smart garment, porous carbon impregnation from the weave, to the yarn, to the fibers. Reproduced from ref. 106 with permission from The Royal Society of Chemistry |
| XIII |  |
The image of the energy supply devices applied to a knit shirt and connected by conductive thread (TEG size: 1.5 cm × 6 cm) Reprinted with permission from ref. 10 |
| XIV |  |
(a) A photograph of wearable textile battery embedded in clothes together with its enlarged view of the inner cell structure, (b) photograph and schematic representation of a watch with a wearable textile battery strap, (c) a schematic illustration of the cell configuration of the wearable textile battery. Reprinted with permission from ref. 13 |
| XV |  |
(a) A fiber cell being woven with the other CNT fibers into a textile and (b) a fiber cell being woven into a textile composed of aramid fibers. Reprinted with permission from ref. 109 |
4.1 E-textile components and other smart electronics
Tao et al.11 Chiolerio et al.112 and Lee et al.115 reviewed and summarized various e-components and their applications because textile-based wearable electronic devices demand simultaneous achievements of electronic functions and robust mechanical properties.11,112,115 Table 4 shows performance, properties of hybrid nanostructured conductive cotton materials for wearable electronics and e-textile components.| Type of material | Name of key material | Important properties and performance | Particular application |
|---|---|---|---|
| CNMs | Cotton fabric/thread coated with CNTs12,33,34 | 11 Ω cm−1,33 5 kΩ,34 high thermal conductivity (0.026–0.065 W m−1 K−1, superior to other synthetic and natural fibers), | Conductive thread as a heater and the fabrication of stretchable wire for lightening the LED,12,33,34 organic transistor12 |
| NMs | NW coated textile thread | Resistance of 0.8 Ω cm−1, sheet resistance of 0.18 Ω cm−2 | |
| Ni, Ag, Cu coated fabric | Antenna gain of 4.4–5.5 dB, frequency band of 2.4 GHz | Textile patch antenna11 |
4.2 Sensors
Sensing technology4,6,12,15–18,82,83,86,105 has become more significant because of various sensing methods and their widespread smart wearable applications. Xiao et al.105 reviewed and summarized the various sensing methods and materials showing their advantages, disadvantages along with their performance.105 Commercial cotton materials coated with CNTs then partially coated with suitable polymeric layers are used as electrical conductors to develop ion-selective electrodes that can sense pH, K+ and NH4+. It is also demonstrated that this approach could be effectively executed on a wearable sensing device.6 The single wired cotton fiber based OECT has been demonstrated to be very effective for electrochemical sensing of NaCl concentration in water, attractive for wearable electronics in fitness and healthcare.16 Similarly, these CNT coated conductive cotton threads can be actualized by designing by two perpendicular Au-wires in contact with CNT–cotton yarn due their good hydrogen bonding, whereas CNTs can be used for the electrode material for NH3 sensing without losing resistance and chemical response as well as the development of low-cost smart sensing devices.4 The e-textile material based sensors are often designed to collect physical biometrics such as electrocardiogram, electromyogram, heart rate, blood pressure, temperature, gathering of biological information from body fluids such as tears, sweat, urine, and blood, detection of dehydration, hypernatremia and glucose level. Another application for e-textile can be chemical sensors for environmental monitoring; e.g. textile fibers coated with CP like PPy, or PAni have been used to test for toxic vapors such as ammonia and nitrous oxide.4 Castano et al.12 and Chiolerio et al.112 reviewed about e-textile technologies and summarized the recent developments in advanced fields of the smart fabric based sensors (e.g. pressure and force sensors, fabric strain sensors, optical fabric sensors, fabric sensors for detection of chemicals and gases, temperature and humidity sensitive fabrics and shape memory fabric sensors). This summary gives an idea about the basic principles and approaches employed while designing fabric sensors as well as the most commonly used materials and techniques used for the development of electronic textiles.12 Tao et al.11 and Lee et al.115 reviewed and reported about fabric-base sensors, many of which have been not only demonstrated as prototypes, but also widely used in real applications of wearable sensing and personal protection. Fiber-based sensors include strain sensors, pressure sensors, chemical sensors, as well as optical and humidity sensors. In this review, Tao et al.11 summarized a comparison of typical fiber-based sensing techniques, advantages, disadvantages and the typical fiber based sensor and sensing networks are presented, e.g., Lycra/cotton fabrics and resistive fabric strain sensors, CNT/cotton fabric piezoresistive strain sensor, CNT/cotton gas sensor, etc. Table 5 shows performance, properties of hybrid nanostructured conductive cotton materials based wearable smart devices for sensors and molecular detection.| Type of material | Name of key material | Particular application | Important properties and performance |
|---|---|---|---|
| Nanomaterials | CNT/cotton threads4 | Ammonia sensors4 | Sheet resistance of 7.8 kΩ cm−1, room temperature sensing, ammonia detection range 5–100 ppm, sensitive at 90° bent and strain up to 14% |
| Cotton/ionic liquid18 | Optoelectronic sensor arrays for chemical detection | More flexible, low volume, and lightweight array to estimate pH and detect a variety of vapors | |
| Hybrid nanostructures and nanocomposites | SWCNT–nylon NFs74 | EMI shielding materials74 | Shielding effectiveness of EMI = 30 dB, tensile strength of 69 MPa |
| Polymer nanocomposites | CNT/cotton threads/PVC membrane6 | Electrochemical sensors for detection of pH, K+ and NH4+ | Sheet resistance of 500 Ω cm−1, conductive, ion-selective yarns, electrochemical detection, optimum response and selectivity, very good reproducibility, easily connectable to reading instruments, low cost sensors, applicable for disposable, wearable devices for LOD of pH, 10 μM K+ and 1 μM NH4+ |
| Cotton/PEDOT:PSS16 | Organic electrochemical transistor for liquid electrolyte, saline (NaCl) sensing | Physiological range for the human sweat (2 × 10−2 to 8 × 10−2 M) to evaluate the suitability of the cotton–OECT device as a sensor for the saline concentration in the human sweat, physiological range of chloride in the sweat (30–60 mM) are clearly distinguishable | |
Cotton threads/CNTs/PAni-Fe2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 123 123 |
LPG sensing at room temperature | Quick response time and maximum response value (Rres = 0.91) were observed for low concentration (50 ppm) detection of LPG at ambient temperature123 |
| Type of material | Name of key material | Important properties and performance | Demerits | Name of wearable device |
|---|---|---|---|---|
| CNMs, nanomaterials | CNT/PSS–water/cotton yarn1,2 | Ultimate yield strength = 41.6 to 87.8 MPa; initial modulus = 140 to 342 MPa; tensile breaking strain = 0.36 to 0.28, respectively, the density-normalized breaking energy is 65 kJ kg−1 | Too high metallic conductance | Biosensor to detect albumin1,2 |
| Hybrids, nanostructures, polymer nanocomposites | Cotton/CNT/PTFE8 | Resistance 0.644 kΩ cm−1, measures physiological and biomechanical signals, human motions, it can charge 10 nF capacitor, power density of 0.1 μW cm−2 | High performance polymer is required for coating of cotton | FBG device for power shirt, wireless body temperature measurement8 |
| PPy/Lycra/cotton fabrics11 | Gauge factor: 80, strain: 50% | Poor stability, durability | Pressure and strain sensor11 | |
| Cotton fiber/PEDOT:PSS32 | For measurement of adrenaline concentration of 10−9 M to 10−3 M | Metal wire is required as gate electrode surface | Human stress monitoring OECT for biosensor32 | |
| Graphene/textile/Pt-NPs36 | Sensitivity of 0.56 mA mM−1 and LOD 0.2 μM for hydrogen peroxide, negligible variation in conductivity, withstand stress of 3.5 MPa with 1.2% elongation | Strategy is required for coating of graphene sheets textile fibre | Glucose biosensor, hydrogen peroxide detection36 | |
| Low cost wax patterned cotton cloth,17 cotton fabric/carbon graphite35 | Improved wicking property, 2D, 3D microfluidic devices to detect BSA,17 portable, low cost, disposable, biocompatible, washable, light weight and mechanically bendable, flexible devices for measurement of lactate concentration of 0.1 to 5 mM,35 response of 0.3169 μA mM−1 and LOD of 0.3 mM | Heat treatment is required,17 FED does not meet equipment criteria, it requires electrochemical analyzer35 | Cotton cloth for performing colorimetric bioassays,17 FED to detect hydrogen peroxide, lactate measurement in saliva, blood, serum and urine35 |
 | ||
| Fig. 14 (a) The resistance response of all metallic CNT sensors. No specific response was found. (b) The resistance response for metallic–sensing–metallic sensor. (c) The NH3 response to plane and bent CNT–cotton yarn stitched on a garment, the influence of bending stress shows negligible effect on sensor response. Reprinted with permission from ref. 4; response of the sensing yarns (d) K+. Reproduced from ref. 6 with permission from The Royal Society of Chemistry. | ||
4.3 Smart garments for healthcare applications
Smart garments for monitoring physiological and biomechanical signals of the human body are key sensors for personalized health care. Wearable devices for healthcare applications like smart shirt can monitor vital signs such as heart rate, body temperature, etc. Highly sensitive, stretchable and wearable multifunctional sensors have been made to detect multiple stimuli such as stretch, strain, pressure, temperature and finger touch with high sensitivity, fast response time and good pressure mapping function. Flexible sensing devices have been reported for several wearable applications including monitoring thumb movement, sensing the strain of the knee joint in patellar reflex (knee-jerk) and other human movements such as walking, running and jumping from squatting, which illustrate their potential utilities of such sensors in robotic systems, prosthetics, healthcare and flexible touch panel.8,9 | ||
| Fig. 15 FBG as a self-powered active sensor for body motion detection: (a) current–time response curve and (b) the corresponding change transfer through an 80 MΩ external load of the FBG that was fixed on an index finger at five different bending releasing finger motion amplitudes, The down insets in (a) labelled as I, II, III, IV, and V demonstrate the five finger motion states. Reprinted with permission from ref. 8. | ||
Hata et al.28 made wearable devices from stretchable electronic materials such thin films of aligned SWCNTs and conventional rigid engineering materials that could be incorporated into wearable textiles (stockings, bandages and gloves) or attached directly to the body. This mechanism allows the films to measure strains up to 280%, with high durability, fast response and low creep to detect human motion, including movement, typing, breathing and speech. A stretchable human motion detector has been fabricated by connecting CNT gel based conductive rubber paste stretchable electrodes to the films as well as PDMS rubber glue and assembling them on wearable cotton textiles. This mechanism is interesting which should be tested on flexible and linear CNT coated conductive cotton threads. Adhesive bandages and SWCNT film device behave as a single cohesive stretchable object, so deformation of the skin can be monitored directly and precisely using the SWCNT film (Fig. 16(a)). When fixed to the chest, respiration could be monitored by the upward and downward slopes of the relative resistance associated with inhalation and exhalation (chest expansion and contraction). In contrast, when attached to the throat (Fig. 16(b), inset), the device monitored phonation (speech) by detecting motion of the laryngeal prominence (Fig. 16(c)). Such devices might be useful in a breathing monitor for the early detection of sudden infant death syndrome in sleeping infants, alerting parents to any potential problems. To detect large-scale human motion, small films can be seamlessly connected to fabricate a large SWCNT strain sensor assembled on a commercial stocking (Fig. 16(d)) over the knee joint. The large SWCNT film was necessary to detect and distinguish every movement of the knee. As the knee joint moves in one direction (as well as swiveling on its axis), the knee constantly rolls and glides during movement, so the deformation site of the skin is constantly varying. Although it was made from just one sensor, the device could easily detect, and also discriminate, various human motions related to the extension and flexion of the knee, including bending, marching, squatting and jumping, and combinations of these (Fig. 16(e)). One advantage of using clothing-integrated devices is the option for repeatable and sharable use of the sensor. Integration of the SWCNT strain devices were created a system for the configuration of the human body, as demonstrated by a data glove made from five independent SWCNT strain sensors assembled on a single glove (Fig. 16(f)). A data glove is an interactive device, resembling a glove normally worn on the hand, which facilitates fine-motion control in robotics and virtual reality. The designed data glove could detect the motion of each finger individually and precisely (Fig. 16(g)), and the output of each gauge could be measured to assess the hand configuration. The designed glove is lighter, simpler, allows integration of more sensors than the complex optical fiber system, and does not limit any range of motion of the hand, as does the metal–strain–gauge system. This device might be used as a master-hand to control a remote slave robot to remotely perform surgical procedures or to increase safety and speed.28
 | ||
| Fig. 16 Photographs of stretchable, wearable (a) bandage strain sensor (d) a strain sensor fixed to a stocking and (f) a data glove, inset to (a): photograph of the sensor adhered to the throat, inset to (d): close-up of the device, (b, c, e, g) relative changes in resistance versus time for breathing, phonation (speech), knee motion and data glove configurations, respectively. Reprinted with permission from ref. 28. | ||
Especially under stress situations and strongly physical conditions, the selective detection of adrenaline with respect to the saline content in human physiological fluids has been also reported. Timely sensing of abnormal adrenaline concentration could be a fingerprint of a pathological situation, like panic or heart attack, or could identify a typical flight, fight and fright response. Moreover, it could be used to monitor athletes, where the control of human physiological performances during competition and training is required. The adrenaline sensing in a complex fluid (human sweat) has been reported for the first time using an innovative system of OECTs (human stress monitoring through an organic cotton-fiber biosensor) as already shown in Table 3(III). The devices were applied for the measurements of real human sweat, which have been recorded in real-time using an electrolyte and monitored the OECT sensing. This innovative device is a useful tool for an in situ and non-invasive analysis of human performances (hydration and stress), finding applications in sports, healthcare and work safety.16,32,37 A textile-based respiratory sensing system made from highly flexible polymeric optical fibers (POFs) that react to applied pressure were integrated into a carrier cotton fabric to form a wearable sensing system (Table 3(VIII)). The feasibility of such a wearable sensor, the setup featuring the best performance was placed on the human torso to measure the respiratory rate. Instead of these POFs, hybrid nanostructured conductive cotton fibers can be also applied. Also such a wearable system enables to keep track of the way of breathing (diaphragmatic, upper costal and mixed) when the sensor is placed at different positions of the torso, which can be utilised as commercial respiratory measurement devices.118
 | ||
| Fig. 17 Scheme of the assembly of a cloth-based ELISA device: three devices were fabricated and the antibody was immobilized with different agents: (1) CAD modified with chitosan and immobilized with GA, (2) immobilization with GA and (3) absorbed (non-immobilized). This was followed by the addition of BSA as a blocking agent, antigen and enzyme–conjugated antibody. The addition of the TMB dye produced a blue color as it reacted with the enzyme-linked antibody while HCl stopped this reaction, providing a yellow color for the colorimetric detection. Reproduced from ref. 93 with permission from The Royal Society of Chemistry. | ||
Córcoles et al.35 designed a simple and low-cost cotton fabric-based electrochemical device (FED) for the determination of lactate concentration (up to limit of 0.3 mM) in saliva. The device is especially useful for clinical diagnostics and sports monitoring. The FED combines the advantages of cotton fabric (easily available, low-cost, lightweight, flexible, biocompatible, requiring min volume of reagent and sample solution, mechanically durable and environment friendly) with the benefits of electrochemical detection (fast and reliable quantitative analysis). The wearable devices were designed by scoured cotton fabrics using Na2CO3 (20 g in 1 L of ultrapure boiled water) followed by washing, rinsing and drying treatment in order to produce a sufficiently hydrophilic cotton fabric. Next, all necessary electrodes for a three-electrode configuration system were integrated into the treated cotton fabric by using template method. The template was printed on self-adhesive vinyl paper using a digital craft cutter (Fig. 18(A)(b)). The printed template was adhered to the cotton fabric surface, then the template openings were filled with carbon graphite paste and Prussian Blue (C-PB) paste for the WE and CE, respectively, while Ag/AgCl paste was used as the RE (Fig. 18(A)(c)). After removing the template, the cotton fabric was cured at 60 °C for 30 min in the oven (Fig. 18(A)(d)). The hydrophilic sample placement/reaction zone was patterned on the electrode-embedded cotton fabric using candellila wax-patterning technique. The templates for the sample placement/reaction zone were designed using software and printed on the wax-impregnated paper (Fig. 18(A)(e)) which was placed accordingly on the cotton fabric and the wax was transferred by heat treatment using a soldering iron at an operating temperature of 150 °C (Fig. 18(A)(f)). When the wax melts, it spreads in both vertical and lateral directions within the cotton fabric. The FED was ready to use after removing the template and allowing it to cool at room temperature (Fig. 18(A)(g)). All the electrochemical measurements were performed after cutting the fabric into (15 mm × 15 mm) strips, each containing the three-electrode set (Fig. 18(B)). The overall fabrication process of the FED is illustrated in Fig. 18(B)(a). The electrochemical behavior of PB, a redox-active compound within the fabricated FED was studied from CV curves by using 4 mL of 0.1 M phosphate buffer solution (PBS). The solution wicks through the cellulose fibres within the cotton fabric and reacts with the entrapped lactate oxidase (LOx) enzyme molecules, hence generating H2O2 that can be electrochemically detected. The reaction that takes place at the C-PB/LOx electrodes of the FED in the presence of lactate are illustrated in Fig. 18(B)(b). The template method was used to pattern a single conventional three-electrode sensor and a three-electrode array onto commonly available lab supplies as shown in Fig. 18(C). The resulting devices could be easily interfaced with an electrochemical analyzer, thus making it feasible for a wide array of applications such as healthcare, clinical diagnostics, sports, agriculture, environmental, security and food quality monitoring.35 Similarly, a fabrication of NaOH-scoured and Na2CO3-scoured cotton cloth-based microfluidic device (CMD) has been reported using a simple wax patterning method for performing calorimetric bioassays. The wax pattern was written by hand or transferred using a metallic stamp onto cotton cloth so that when the cloth is dipped in dye, the dye will not penetrate the region which is covered with wax. Hot melted wax was applied to fill in the gaps between the fibers in a single yarn as well as in the space of cotton fabrics, which create hydrophobic regions in a hydrophilic substrate (Fig. 19(I)(A–C)). Fig. 19(I)(B) shows a magnified SEM image of the unwaxed region with clear gaps between the single cotton fibers, while in Fig. 19(I)(C) showing magnified SEM image of the waxed region with gaps as well as the surface of individual fibers covered with wax. Fig. 19(I)(D) shows more proof from a light microscopy image that wax covers both the weave porosity and the gaps between the fibers. Yet, the gaps between the fibers in a single yarn appear darker compared to those of the waxed region. The increased hydrophobicity of the waxed region is mainly due to the increased content of long aliphatic chains of the fatty acids contained in the wax. This is proven by the extreme increase of the C atom content in the wax region compared to the non-waxed region, as shown in Fig. 19(II)(E).17 The colorimetric detection of protein was utilized as a model assay to examine the function of 2D and 3D CMDs (Fig. 19(II)) as an analytical device which was used to detect unknown amounts of BSA in artificial urine. The white cotton fabric has enough contrast with the blue coloration resulting from positive BSA samples (Fig. 19(II)(B), (F) and (H)). The results demonstrated that these CMDs can be utilized for diagnostic application by performing colorimetric assays of body fluid samples. Fig. 19(II)(G and H) particularly prove that the assay can also be carried out in a bent cloth platform.17
 | ||
| Fig. 18 (A) Schematic illustration of the fabrication process of the FED: (a) the platform for FED treated cotton fabric, (b) for patterning the electrodes, self-adhesive vinyl template was used, (c) C-PB paste was applied for both the WE and CE, while Ag/AgCl paste was applied for the RE, (d) after the template was removed, the substrate was cured at 60 °C for 30 min in the oven, (e) the template for patterning the sample placement/reaction zone was printed on wax-impregnated paper, (f) the wax-impregnated paper template was placed accordingly and heat treatment was used to transfer the wax onto the substrate at 150 °C using a soldering iron, (g) the ready-to-use device: RE, WE and CE; (B) overview of FED technology: (a) the instrumental setup for lactate determination, (b) the reaction that occurs at the C-PB/LOx electrodes of the FED, (c) picture of the fabricated FED (15 × 15 mm): RE, WE and CE, (C) electrodes patterned on: (a) glass microscope slide, (b) cotton fabric, (c) plastic weighing boat, (d) on the outer surface of a poly-propylene centrifuge tube, (e) nitrile glove. Reproduced from ref. 35 with permission from The Royal Society of Chemistry. | ||
 | ||
| Fig. 19 (I) SEM image of the boundary (shown by a dotted line) between untreated and wax-treated areas of cotton cloth (A) at 50 magnification with 500 mm scale bar, zoom-in image of (B) area without wax, (C) wax region with 50 mm scale bar. Microscopy image of the two regions is shown in (D), the gaps between the fibers in the nonwax region absorb transmitted light (D-bottom), while after being filled with wax, the light is diffusely transmitted (D-top), (E) EDS of cotton cloth at the boundary between wax and non wax regions; (II) 2D and 3D CMDs for running colorimetric protein assays of artificial urine samples: (A) for the control sample (i.e. no protein), (B) for positive sample (i.e. with protein), (C) the 3D CMD before folding, (D) the top and (E and F) bottom sides of the 3D CMDs after adding 5 mL of control (E) and positive (F) samples, respectively. The results of assays in bent flexible 2D CMDs are shown (G) for control sample and (H) for positive sample. All devices are designed with the same channel width size (less than 1 mm). Reproduced from ref. 17 with permission from The Royal Society of Chemistry. | ||
Glucose meters are well-established over-the-counter devices that allow monitoring of blood glucose concentration. Cotton thread based scanners, smartphone apps and plug-in devices are now available point-of-care diagnostics with consumer electronic devices, according to review reported by Algar et al.81 Glucose detection has been also demonstrated by graphene/textile based flexible and stretchable composites (conductivity = 0.58 S cm−1) with sensitivity of 150.8 μA mM−1 cm−2 and a low LOD of 1 μM (S/N = 3).36
4.4 Energy devices
To meet the rapid development of flexible, portable, and wearable smart devices, extensive efforts have been devoted to develop matchable energy storage and conversion systems as power sources, such as SCs, LIBs, solar cells, fuel cells, etc.18,19 Various research groups (Tao et al.11 Zhang et al.19 Shen et al.30 Shi et al.20 and Lee et al.115) reviewed and summarized various wearable energy harvesting, conversion and storage devices which include SCs electrodes, transparent SCs, wearable SCs transistors, flexible and fibrous dye-sensitized solar cells (DSSCs), generators, integrated devices and systems and energy converters, photovoltaic devices, fuel cells, nanogenerators, SCs, LIBs, OLEDs and photo detector cum actuators. The use of active pseudo capacitive materials and permeable cotton material based conductive textiles is also a useful platform for energy storage device applications. Cotton based textiles are highly porous and can absorb large amounts of water and other polar solvents, which can be made conductive by an extremely simple “dipping and drying” process using CNT ink. These everyday textile base wearable power devices have demonstrated outstanding flexibility, stretchability owing to strong adhesion between the CNTs and cotton based textiles due to VdWs interactions. Table 2 shows various cotton based wearable devices and smart textiles for energy management applications.Recently, cable-type devices have been designed with new concept of device architecture, which can maximize the mechanical flexibility and provide the breakthrough necessary as wearable devices in the field of energy conversion and storage. Due to perfect bending properties that these devices could meet the requirements of wearable energy devices and such device can be woven into any shape and placed anywhere. Gao et al.5 prepared a novel high-performance, cable-type SC (Table 3(X)) based on multi-grade 3D nanostructures of PPy–MnO2–CNT-cotton thread via an EDM. Porous functionalized cotton threads were used as both current collectors and active charge storage electrodes in SC testing and transparent silicone pipeline was used as a package shell, the assembly of a fully cable-type SC was accomplished.5 Multiple SCs connected in series and in parallel at highly twisting and bending states can successfully drive an LED segment display (it can be operated with a 1.68 V voltage and a 4 mA current), illustrating its superior performance (high capacitance, stable cycling life, remarkable flexibility, high energy and power density).5
Zhu et al.22 used about CFs (140–160 mm diameter) electrochemically deposited with ERGO and characterized as symmetric SC electrodes. This string-like solid SC is flexible enough to be easily woven into cotton textiles. The total length specific capacitance of ERGO@CF–; H/PVA–H3PO4 based SCs was reported to be up to ∼13.5 mF cm−1, while low equivalent series resistance (ESR) of ∼5 Ω cm−1 was obtained from the Nyquist plot. Energy density and power density are two key parameters which determine the quality of a capacitor, which were reported to be ∼1.9 mW h cm−1 at a power density of 27.2 μW cm−1 and the maximum power density was 748.6 μW cm−1 without a great loss of energy density.22 A asymmetric SCs (ASCs) based on acicular Co9S8 nanorod arrays grown on carbon cloth as positive materials and Co3O4@RuO2 nano-sheet arrays as negative materials have been reported.30,104 In another study, SC was made of two pieces of MnO2/CNT fabric electrodes sandwiched with H3PO4/PVA solid-state electrolyte with tandem stack and laminated configurations. To enhance the capacity, pseudo capacitive materials such as ZnO–MnO2–NRs arrays, ZnCo2O4-NWs and PAni-NWs have been elaborated on these fibers to develop fiber-shaped SCs which can work independently or be woven into wearable textiles.30 A plannar shaped fiber SC has been prepared by twisting two CNT@PAni single yarns with the PVA gel electrolyte. A model fabric was composed of four conventional two-ply cotton yarns and four two-ply CNT@PAni@PVA yarns, giving a prototype of woven energy devices with a capacitance of 38 mF cm−2 at a current density of 0.01 mA cm−2.25,30
Gao et al.7 developed cable-type SC devices using polyelectrolyte-wrapped graphene/CNT core-sheath fibers (Table 3(XI)) by maximizing the mechanical flexibility and impeccable bending properties in wearable devices for the application of energy conversion and storage. The GO–liquid crystals (LC) and the CMC aqueous solution as an electrically insulative polyelectrolyte were used to prepare polymer wrapped GF which can be woven into cloth to form a bendable SC. The rGO@CMC fibers were highly conductive, having a conductivity of 70 S cm−1. Image (a) in Table 3(XI) shows a co-woven cloth using multiple cotton yarns and two intact flexible coaxial fibers that are without fracture, as demonstrated by the optical microscopy image of the co-woven cloth (image b in Table 3(XI)). Two individual 40 cm-long coaxial rGO-CNT@CMC fibers as anode and cathode to interweave a cloth SC (image c and d in Table 3(XI)). The yarn SCs using liquid and solid electrolytes show ultra-high capacitances of 269 and 177 mF cm−2 and energy densities of 5.91 and 3.84 mW h cm−2, respectively. These types of YSCs have extraordinary potential and these cloth SCs interwoven from individual intact fiber electrodes have been reported.7,20 Wang et al.20 reviewed various flexible SCs and reported about flexible graphene electrodes prepared by simply brush-coating of GO inks on cotton cloth and followed by annealing. The flexible SC with these electrodes showed a specific capacitance of 81.7 F g−1. The GF electrodes can be further assembled into a yarn SC, showing a high specific capacitance of 409 F g−1. The ultra-elastic GF based flexible electrodes can also be prepared by depositing graphene materials on flexible substrates such as sponges for various wearable electronics in large scale.20 Apart from the significant progress of CNT- and graphene based SCs, other carbon-based flexible SCs fabricated using uniformly screen printed porous carbon on cotton textiles, (Table 3(XI)) have been reported by Gogosti et al.19,26,106 The analysis of cotton textiles based electrodes was carried out by CV and GCD analysis to study the capacitive behavior of carbon materials using nontoxic aqueous electrolytes including sodium sulfate and lithium sulfate. The capacitive behavior of cotton lawn electrodes was less resistive and CV curves were rectangular in both electrolytes. The CA of cotton lawn in sodium sulfate drops from 0.43 F cm−2 at 1 mV s−1 to 0.37 F cm−2 at 100 mV s−1. Equivalent series resistance (ESR) was reported to be 3–4 Ω cm−2 obtained from impedance spectroscopy and GCD curves for cotton lawn in both electrolytes. Cotton lawn electrodes exhibited high specific capacitance from GCD curves, average gravimetric capacitances of 85 F g−1 at 0.25 A g−1, average areal capacitance of 0.43 F cm−2 at 5 mA cm−2 due to their similar masses.106 Textiles integrated with omnidirectional, flexible and even twistable well-designed wire shaped SC (WSSCs) composed of two fiber electrodes, a helical space wire, an electrolyte, and a plastic tube outer package can be fabricated into woven into any shape of clothes to power electronic devices. The resulting WSSC prepared using a commercial pen ink exhibited a good areal capacitance of 9.5 mF cm−2 and a stable cycling performance over 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.19,107 Sponges with macroporous nature were used as flexible substrates to load MnO2 as pseudocapacitance active materials, which showed many excellent properties such as high theoretical specific capacitance (1400 F g−1), low cost, low toxicity and natural abundance. The highly conductive and porous MnO2/CNT/sponge based SC was reported to be stable pseudocapacitance and double layer capacitance, a high specific capacitance of 1230 F g−1, a specific power density of 63 kW kg−1, a specific energy density of 31 kW h kg−1. The MnO2-CNT/sponge exhibited excellent performance as SC, making it a promising electrode for future energy storage wearable systems.19,21 A thread-like planar-shaped SC built on woven individual fibers or parallel arranged fibers has been demonstrated for a high-performance. By using the CNT coating on the common cellulose fibers or cotton materials, robust and flexible electrodes can be developed. To enhance the capacity, pseudo capacitive materials such as ZnO–MnO2–NRs arrays, ZnCo2O4-NWs and PAni-NWs have been incorporated on these fibers. Fiber-shaped ZnCo2O4-NWs on carbon cloth based SCs can be woven into any desired shape and a two-ply composite yarn consisting of two CNT single yarns can be also infiltrated with PAni-NW arrays.111
000 cycles.19,107 Sponges with macroporous nature were used as flexible substrates to load MnO2 as pseudocapacitance active materials, which showed many excellent properties such as high theoretical specific capacitance (1400 F g−1), low cost, low toxicity and natural abundance. The highly conductive and porous MnO2/CNT/sponge based SC was reported to be stable pseudocapacitance and double layer capacitance, a high specific capacitance of 1230 F g−1, a specific power density of 63 kW kg−1, a specific energy density of 31 kW h kg−1. The MnO2-CNT/sponge exhibited excellent performance as SC, making it a promising electrode for future energy storage wearable systems.19,21 A thread-like planar-shaped SC built on woven individual fibers or parallel arranged fibers has been demonstrated for a high-performance. By using the CNT coating on the common cellulose fibers or cotton materials, robust and flexible electrodes can be developed. To enhance the capacity, pseudo capacitive materials such as ZnO–MnO2–NRs arrays, ZnCo2O4-NWs and PAni-NWs have been incorporated on these fibers. Fiber-shaped ZnCo2O4-NWs on carbon cloth based SCs can be woven into any desired shape and a two-ply composite yarn consisting of two CNT single yarns can be also infiltrated with PAni-NW arrays.111
A triboelectric generator (TEG) can be used with wearable electronics because it is one of the promising options for an energy harvesting device because they are sensitive to humidity, exposed to mechanical damage by friction and discontinuous in power generation. TEG based devices were fabricated on a conductive carbon fabric, which allows them to be woven onto designated locations of conventional clothing, and interconnected by conductive threads.10 Kim et al.10 presented a fabric based wearable integrated energy device, consisting of TEGs combined with SCs, which can be utilized either as an activity monitor or as power supply for other wearable sensors. The fully integrated wearable energy device is shown in Table 3(XIII). In this, the fabric-based TEGs (5 cm × 9 cm, 18 lines) and SCs were easily sewn into commercial clothing items, such as a shirt, and then connected by conductive carbon threads (Table 3(XIII)). The energy harvest through regular daily activities such as running and walking was simulated by rubbing the TEGs at various speeds. At a speed of 1.5 Hz, the average output voltage and the rectified current were measured to be 33 V and 0.25 μA; the generated electricity stored in SCs was powerful enough to light up an LED. Regarding the angular motion of the human arm swing, the power generation efficiency can be improved by adjusting the design of TEGs. In addition to monitoring the activity, the SCs charged by TEGs can also supply power to other sensors. To demonstrate this, SCs charged by the TEGs were used to provide the necessary current to a pressure sensor consisted of a porous pressure-sensitive rubber sandwiched between CFs. The structure and principle of operation of the wearable energy generating system are also depicted schematically in Fig. 20. As shown in Fig. 20(a), the TEGs were positioned in the armpit region to maximize friction, whereas the SC was located on the chest section, a region that is safe from friction damage, yet still close to the TEGs. The typical swinging motion generated during walking and the corresponding electricity generation and storage are also shown. A circuit diagram of the integrated energy supply devices is provided in Fig. 20(b), wherein multiple TEGs are connected in parallel to generate sufficient electrical current to charge the SCs. For the inner side of the arm, PU and PI were alternatingly patterned on carbon fabric to form what is hereafter referred to as TEG I (Fig. 20(c)). On the opposite surface, a similar patterning of PDMS and Al was used to create TEG II (Fig. 20(d)). The generated electricity was stored in the integrated fabric-based SC, which has a symmetric structure (CF/CNT/RuO2 electrode – PVA/H3PO4 gel electrolyte – CF/CNT/RuO2 electrode) as shown in Fig. 20(e).10
 | ||
| Fig. 20 Schematic descriptions and morphology of the TEG and SC: (a) schematic illustration of arm swings with TEG and SC equipped, (b) circuit diagram of the integrated energy supply devices, schematic illustrations of SC dual components and digital photos of individual components: (c) TEG I, (d) TEG II, and (e) SC. Insets showing AFM images. Reprinted with permission from ref. 10. | ||
4.5 Other devices
Following are the previously reported devices which have been fabricated using flexible and linear cotton materials functionalized by various nanostructures. | ||
| Fig. 21 (a) SEM image of carbon nanospheres, (b) AFM image of GO nanosheets on silicon, (c) schematic illustration of the hierarchical nanostructured FFSC electrode, (d) the FFSC electrode based on hierarchical composite containing GO nanosheets and carbon nanospheres, with a unique self-assembled LbL structure, SEM image of the as prepared FFSC electrode: (e) side-view, and (f) cross-sectional view, the inset of (f) is the zoom-in SEM image of the edge section of the as-constructed porous carbon sphere/GO structure. Reproduced from ref. 23 with permission from The Royal Society of Chemistry. | ||
 | ||
| Fig. 22 (a) Schematic diagram illustrating the fabricating process of an FBG, SEM images of a CCT with (b) low and (c) high magnification, respectively, SEM images PCCT with (d) low and (e) high magnification, respectively, digital photograph of FBGs: (f) with linear shape, (g) with curved shape, and (h) woven into fabric. Reprinted with permission from ref. 8. | ||
5 Conclusion and outlooks
In this critical review, we have covered recent research on the materials, fabrication and properties of nanostructured flexible and linear cotton materials (threads, fibers, yarns and fabrics) and their applications in wearable devices related to e-textile components (transistors, antenna, electrical connectors, fiber based electric circuit), energy management (conversion, LIBS, DSSCs, FFSCs, LEDs, energy generators, fuel cells), sensors (biomolecules detectors, gas, chemical, electrochemical, pH, stress–strain, pigment and dyeing), actuators, healthcare garments (human body movement, diagnosis, glucose detecting fabrics), smart textiles and flexible electronics (power shirt, FEG, TEG, flexible heater). The complete review of hybrid nanostructured cotton threads shows the following concluding remarks.The conductive cotton based textiles materials can offer here a uniquely simple yet remarkably functional solution for wearable and smart devices, with many parameters exceeding the existing technological solutions, including different conductive fillers (CNMs such as carbon particles, CFs, CNTs, GO, rGO, graphene, GFs, metals and metal oxide NPs, NWs, hybrids, nanocomposites, CPs, CP based nanocomposites). The CNM–cotton materials are promising for low-cost e-textile based devices for applications in sensors, biosensors, heaters, and healthcare monitoring clothes due their electrical properties, while NMs (i.e., NPs, NWs)-cotton materials are found suitable for wearable electronics due their optical properties. Similarly, hybrids, nanocomposites and CP nanocomposites are suitable conducting fillers for cotton material based wearable devices for energy management applications. The hydrogen bonding between the CNMs and cotton leads to high adhesion, as a result, conductive cotton material can be implemented to develop smart and wearable devices. Flexible and linear cotton materials (extruded threads, fibers, yarns, fabrics) can be made conductive using aformentioned conducting fillers by various methods during manufacturing and after manufacturing (galvanic deposition”, “atomic layer deposition”, “electrochemical deposition”, “EDM”, film coating”, “screen printing”, “silk screening”, “sputtering”, “electroless plating”, “CVD”, “vapor coating”, “dipping and drying”, “self assembly”, “epitaxial growth”, “chemical reduction”, “pulsed laser deposition”). Amongst them, “dipping and drying” method has been most widely used to make conductive cotton materials, while “electrochemical deposition method” was used for depositing the energetic materials. The as-made conductive cotton materials are flexible and robust enough to be intertwined, knotted and woven into clothes, garments and hand accessories. In addition, these flexible and linear materials can also be used as conductive cables, wires, connectors, antennas, with engineering garments. On the other hand, these conductive cotton materials based components can be equipped with tiny self-powered elements and microwave communication components to serve as body-implantable sensing networks, roll-up portable displays, sensory skins and electronically steerable antenna arrays for wireless communication, would be in considerable demand in upcoming years. Wearable devices such as highly bendable cloth sensor, intelligent micro sensors, wearable electronics, “power shirt”, FEG, TEG, a cable-type SCs, LIBs, DSSCs, scanners, diagnostic devices, antimicrobial textiles and many more.
Furthermore, nanostructured conductive cotton material-based wearable devices were explained for various applications. To commercialize nanostructured conductive cotton material in wearable devices, some important issues will have to be addressed, including mass production, integration into clothes, non-toxic technology, and long-term usage. Although considerable performance in textile-based electronic devices have already been achieved, further efforts to improve performance are necessary. A significant body of theoretical and experimental research has been carried out to understand the mechanism and characteristics of smart e-textiles and wearable devices. Current trends suggest that smart and wearable devices are well-positioned to someday become the foremost wearable devices for different applications in worldwide. These devices are exceedingly popular in younger demographics, and can function as an all-in-one tool for measurement, processing and communication of results. If the e-textiles can overcome the aforementioned issues, new era in wearable devices will begin.
This review also promises to be an increasingly active area of research for the foreseeable future and advances in wearable technology and shows potential to have a profoundly positive impact on quality of next generation life. These recent advances in the development of wearable devices and smart textiles will bring for future devices into a realization. The overview of hybrid nanostructured cotton materials will boost essential encouragement for the development of next generation smart textiles and flexible devices which could be worn by human beings.
List of abbreviations
| 1D, 2D, 3D | One-, two- and three-dimensional |
| ACF | Activated carbon fabric |
| AD | Analog digital |
| AFM | Atomic force microscope |
| Ag | Silver |
| AgCl | Silver chloride |
| Al | Aluminum |
| ASCs | Asymmetric super capacitors |
| Au | Gold |
| BSA | Bovine serum albumin |
| CADs | Cloth-based analytical devices |
| CA | Areal capacitance |
| CCT | CNT coated thread |
| Cd | Cadmium |
| CE | Counter electrode |
| CFs | Carbon fibers |
| CH4 | Methane |
| CL | Length capacitance |
| cm | Centimeter |
| CMC | Carboxy methyl cellulose |
| CMD | Cloth-based microfluidic device |
| CMFs | Carbon microfibers |
| CNMs | Carbon nanomaterials |
| CNTs | Carbon nanotubes |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| C-PB | Carbon graphite paste modified with prussian blue |
| CPs | Conductive polymers |
| CRP | C-reactive protein |
| CuO | Copper oxide |
| CV | Cyclic voltammetry |
| CV | Volume capacitance |
| CVD | Chemical vapor deposition |
| DBSA | Dodecyl benzene sulfonic acid |
| DC | Direct current |
| DCM | Dichloro methane |
| DI | De-ionized |
| DMSO | Di methyl sulfo-oxide |
| DSSC | Dye-sensitized solar cells |
| EDLCs | Electrical double-layer capacitors |
| EDM | Electroless deposition method |
| EDS | Elemental analysis |
| ELD | Electroless deposition |
| ELISA | Enzyme-linked immunosorbent assay |
| EMF | Electro motive force |
| EMI | Electromagnetic interference |
| ERGO | Electrochemically reduced graphene oxide |
| ESR | Equivalent series resistance |
| FBG | Fiber-based generator |
| FeCl3 | Ferric chloride |
| Fe2O3 | Iron oxides |
| FED | Fabric-based electrochemical device |
| FFSC | Fibrous, flexible supercapacitor |
| FESEM | Field emission scanning electron microscope |
| GA | Glutar aldehyde |
| GCD | Galvanostatic charge–discharge |
| GCHN | GO nanosheet and carbon nanospheres hierarchical nanostructure |
| GFs | Graphene fibers |
| GO | Graphene oxide |
| H2 | Hydrogen |
| HNO3 | Nitric acid |
| H2O2 | Hydrogen peroxide |
| H2S | Hydrogen sulfide |
| H2SO4 | Sulfuric acid |
| H3PO4 | Phosphoric acid |
| HCl | Hydrochloric acid |
| hCG | Human chorionic gonadotropin |
| HSA | Human serum albumin |
| ICPs | Intrinsically conductive polymers |
| IL | Ionic liquid |
| ISO | International organization for standardization |
| KMnO4 | Potassium dichromate |
| KOH | Potassium hydroxide |
| KPa | Kilo pascal |
| LbL | Layer by layer |
| LC | Liquid crystals |
| LCD | Liquid crystalline display |
| LED | Light emitting diode |
| LFP | LiFePO4 |
| LIBs | Lithium ion batteries |
| LiClO4 | Lithium perchlorate |
| Li2SO4 | Lithium sulfate |
| LOD | Limits of detection |
| LOx | Lactate oxidase |
| LPG | Liquefied petroleum gas |
| LTO | Li4Ti5O12 |
| MCU | Microcontroller unit |
| MgO | Magnesium oxide |
| mm | Milli meter |
| MnO2 | Manganese dioxide |
| Mn(NO3)2 | Manganese nitrate |
| MPa | Mega pascal |
| MWCNTs | Multi walled carbon nanotubes |
| NaCl | Sodium chloride |
| NaClO4 | Sodium chlorate |
| Na2CO3 | Sodium carbonate |
| NADH | β-Nicotinamide adenine dinucleotide |
| NaF | Sodium floride |
| NaNO3 | Sodium nitrate |
| NaOH | Sodium hydroxide |
| NFs | Nano fibers |
| N2H4 | Hydrazine |
| NH3 | Ammonia |
| Ni | Nickel |
| nm | Nano meter |
| NMs | Nano materials |
| NO2 | Nitrogen dioxide |
| NPs | Nano particles |
| NWs | Nano wires |
| OECT | Organic electrochemical transistor |
| OFET | Organic field-effect transistor |
| OMC | Ordered mesoporous carbon |
| P2O5 | Phosphorous pentoxide |
| P3HT | Poly(3-hexyl thiophene) |
| PAni | Poly aniline |
| PAni-NFs | Poly aniline nanofibers |
| PBS | Phosphate buffer solution |
| PCCT | PTFE coated CNT thread |
| PDMS | Poly di methyl siloxane |
| PdCl4 | Palladium chloride |
| PEDOT | Poly(3,4-ethylene di oxy thiophene) |
| PEO | Poly(ethylene oxide) |
| PET | Poly ethylene terephthalate |
| PI | Poly imide |
| Pt | Platinum |
| PMMA | Poly(methyl metha acrylate) |
| POFs | Polymeric optical fibres |
| PP | Poly propylene |
| PPy | Poly pyrrole |
| PSS | Poly(styrene sulfonate) |
| PTFE | Poly(tetra floro ethylene) |
| PThi | Poly thiophene |
| PU | Poly urethane |
| PVA | Poly(vinyl alcohol) |
| PVC | Poly(vinyl chloride) |
| PVDF | Poly(vinyliden fluoride) |
| P(METAC-co-MPTS) | Poly[2-(methacryloyloxy) ethyl trimethyl ammonium chloride-co-3-(trimethoxysilyl) propyl methacrylate] |
| PVP | Poly(4-vinyl phenol) |
| rGO | Reduced GO |
| Rh | Rhodium |
| RE | Reference electrode |
| RuO2 | Ruthenium dioxide |
| SCE | Saturated calomel electrode |
| SCs | Super capacitors |
| SDBS | Sodium dodecyl benzene sulfonate |
| SDS | Sodium dodecyl sulfide |
| SEM | Scanning electron microscopy |
| SiO2 | Silicon dioxide |
| SnO2 | Tin oxides |
| SWCNTs | Single walled carbon nanotubes |
| TEG | Tribo electric generator |
| TEM | Tunneling electron microscopy |
| TiO2 | Titanium dioxide |
| TLC | Thin layer chromatography |
| TMB | Tetra methyl benzidine |
| UV | Ultra-violet |
| VdWs | Van der Waals |
| VLS | Vapor–liquid–Solid |
| VOC | Volatile organic compounds |
| VS | Vapor–solid |
| WE | Working electrode |
| WECT | Wire electrochemical transistors |
| WO | Tungsten oxide |
| WSSC | Wire shaped super capacitor |
| YSCs | Yarn super capacitors |
| ZnO | Zinc oxide |
Acknowledgements
Authors are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India (Project no.: 02(0023)/11/EMR-II) for providing financial assistance to carry out the major research project.References
- B. S. Shim, W. Chen, C. Doty, C. Xu and N. A. Kotov, Nano Lett., 2008, 8, 4151–4157, DOI:10.1021/nl801495p
.
- B. S. Shim, Ph.D. thesis, The University of Michigan, 2009, http://deepblue.lib.umich.edu/bitstream/handle/2027.42/62251/bshim_1.pdf?sequence=1
.
- L. Hu, M. Pasta, F. L. Mantia, L. Cui, S. Jeong, H. Dawn, D. Jang, W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708–714, DOI:10.1021/nl903949m
.
- J. W. Han, B. Kim, J. Li and M. Meyyappan, Appl. Phys. Lett., 2013, 102, 1–4, DOI:10.1063/1.4805025
.
- N. Liu, W. Ma, J. Tao, X. Zhang, J. Su, L. Li, C. Yang, Y. Gao, D. Golberg and Y. Bando, Adv. Mater., 2013, 25, 4925–4931, DOI:10.1002/adma.201301311
.
- T. Guinovart, M. Parrilla, G. A. Crespo, F. X. Rius and F. J. Andrade, Analyst, 2013, 138, 5208–5215, 10.1039/c3an00710c
.
- L. Kou, T. Huang, B. Zheng, Y. Han, X. Zhao, K. Gopalsamy, H. Sun and C. Gao, Nat. Commun., 2014, 5, 1–10, DOI:10.1038/ncomms4754
.
- J. Zhong, Y. Zhang, Q. Zhong, Q. Hu, B. Hu, Z. L. Wang and J. Zhou, ACS Nano, 2014, 8, 6273–6280, DOI:10.1021/nn501732z
.
- S. Yao and Y. Zhu, Nanoscale, 2014, 6, 2345–2352, 10.1039/c3nr05496a
.
- S. Jung, J. Lee, T. Hyeon, M. Lee and D. H. Kim, Adv. Mater., 2014, 26, 6329–6334, DOI:10.1002/adma.201402439
.
- W. Zeng, L. Shu, Q. Li, S. Chen, F. Wang and X. M. Tao, Adv. Mater., 2014, 26, 5310–5336, DOI:10.1002/adma.201400633
.
- L. M. Castano and A. B. Flatau, Smart Mater. Struct., 2014, 23, 1–27, DOI:10.1088/0964-1726/23/5/053001
.
- Y. H. Lee, J. S. Kim, J. Noh, I. Lee, H. J. Kim, S. Choi, J. Seo, S. Jeon, S. Kim, J. Y. Lee and J. W. Choi, Nano Lett., 2013, 13, 5753–5761, DOI:10.1021/nl403860k
.
- Q. Zhao, M. B. Nardelli, W. Lu and J. Bernholc, Nano Lett., 2005, 5, 847–851, DOI:10.1021/nl050167w
.
- P. M. Abbasi and S. R. Ghaffarian, RSC Adv., 2014, 4, 30906–30913, 10.1039/c4ra04197f
.
- G. Tarabella, M. Villani, D. Calestani, R. Mosca, S. Iannotta, A. Zappettini and N. Copped, J. Mater. Chem., 2012, 22, 23830–23834, 10.1039/c2jm34898e
.
- A. Nilghaz, D. H. B. Wicaksono, D. Gustiono, F. A. A. Majid, E. Supriyanto and M. R. A. Kadir, Lab Chip, 2012, 12, 209–218, 10.1039/c1lc20764d
.
- W. Indika, S. Galpothdeniya, K. S. McCarter, S. L. De Rooy, B. P. Regmi, S. Das, F. Hasan, A. Taggea and I. M. Warner, RSC Adv., 2014, 4, 7225–7234, 10.1039/c3ra47518b
.
- L. Li, Z. Wu, S. Yuan and X. B. Zhang, Energy Environ. Sci., 2014, 7, 2101–2122, 10.1039/c4ee00318g
.
- X. Wang and G. Shi, Energy Environ. Sci., 2015, 8, 790–823, 10.1039/c4ee03685a
.
- W. Chen, R. B. Rakhi, L. B. Hu, X. Xie, Y. Cui and N. H. Alshareef, Nano Lett., 2011, 11, 5165, DOI:10.1021/nl2023433
.
- Y. Cao, M. Zhu, P. Li, R. Zhang, X. Li, Q. Gong, K. Wang, M. Zhong, D. Wu, F. Linc and H. Zhu, Phys. Chem. Chem. Phys., 2013, 15, 19550, 10.1039/c3cp54017k
.
- X. Zhang, Y. Lai, M. Ge, Y. Zheng, K. Q. Zhang and Z. Lin, J. Mater. Chem. A, 2015, 3(24), 12761–2768, 10.1039/c5ta03252k
.
- J. Xu, H. Wu, C. Xu, H. Huang, L. Lu, G. Ding, H. Wang, D. Liu, G. Shen, D. Li and X. Chen, Chem.–Eur. J., 2013, 19, 6451–6458, DOI:10.1002/chem.201204571
.
- K. Wang, Q. Meng, Y. Zhang, Z. Wei and M. Miao, Adv. Mater., 2013, 25, 1494–1498, DOI:10.1002/adma.201204598
.
- X. Cai, M. Peng, X. Yu, Y. Fu and D. Zou, J. Mater. Chem. C, 2014, 2, 1184–1200, 10.1039/c3tc31706d
.
- G. Zhou, F. Li and H. M. Cheng, Energy Environ. Sci., 2014, 7, 1307–1338, 10.1039/c3ee43182g
.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. I. Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296–301, DOI:10.1038/nnano.2011.36
.
- M. Melzer, J. I. Mönch, D. Makarov, Y. Zabila, G. Santiago, C. Bermúdez, D. Karnaushenko, S. Baunack, F. Bahr, C. Yan, M. Kaltenbrunner and O. G. Schmidt, Adv. Mater., 2014, 27, 1274–1280, DOI:10.1002/adma.201405027
.
- Z. Liu, J. Xu, D. Chen and G. Shen, Chem. Soc. Rev., 2015, 44, 161–192, 10.1039/c4cs00116h
.
- L. Shen, B. Ding, P. Nie, G. Cao and X. Zhang, Adv. Energy Mater., 2013, 3, 1484–1489, DOI:10.1002/aenm.201300456
.
- N. Copped, G. Tarabella, M. Villani, D. Calestani, S. Iannotta and A. Zappettini, J. Mater. Chem. B, 2014, 2, 5620–5626, 10.1039/c4tb00317a
.
- Y. Atwa, N. Maheshwari and I. A. Goldthorpe, J. Mater. Chem. C, 2015, 3, 3908–3912, 10.1039/c5tc00380f
.
- P. Ilanchezhiyan, A. S. Zakirova, G. M. Kumara, S. U. Yuldasheva, H. D. Choa, T. W. Kang and A. T. Mamadalimov, RSC Adv., 2015, 5, 10697–10702, 10.1039/c4ra10667a
.
- R. S. P. Malon, K. Y. Chua, D. H. B. Wicaksonoa and E. P. Córcoles, Analyst, 2014, 139, 3009–3016, 10.1039/c4an00201f
.
- B. Liang, L. Fang, Y. Hu, G. Yang, Q. Zhu and X. Ye, Nanoscale, 2014, 6, 4264–4274, 10.1039/c3nr06057h
.
- S. Hou, Z. Lv, H. Wu, X. Cai, Z. Chu, Y. Zou and D. Zou, J. Mater. Chem., 2012, 22, 6549–6552, 10.1039/c2jm16773e
.
- V. N. Popov, Mater. Sci. Eng., R, 2004, 43, 61–102, DOI:10.1016/j.mser.2003.10.001
.
- C. E. Baddour and C. Briens, Int. J. Chem. React. Eng., 2005, 3, 20–22, DOI:10.2202/1542-6580.1279
.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32–45, DOI:10.1016/j.snb.2012.11.014
.
- D. Jariwala, V. K. Sangwan, L. J. Lauhon, T. J. Marksa and M. C. Hersam, Chem. Soc. Rev., 2013, 42, 2824–2860, 10.1039/c2cs35335k
.
- X. Mao, G. C. Rutledge and T. A. Hatton, Nano Today, 2014, 9, 405–432, DOI:10.1016/j.nantod.2014.06.011
.
- S. Mao, G. Lu and J. Chen, J. Mater. Chem. A, 2014, 2, 5573–5579, 10.1039/c3ta13823b
.
- Y. Li, Y. Shang, X. He, Q. Peng, S. Du, E. Shi, S. Wu, Z. Li, P. Li and A. Cao, ACS Nano, 2013, 7, 8128–8135, DOI:10.1021/nn403400c
.
- D. Kundu, C. Hazra, A. Chatterjee, A. Chaudhari and S. Mishra, J. Photochem. Photobiol., B, 2014, 140, 194–204, DOI:10.1016/j.jphotobiol.2014.08.001
.
- B. A. Cakır, L. Budama, O. Topel and N. Hoda, Colloids Surf., A, 2012, 414, 132–139, DOI:10.1016/j.colsurfa.2012.08.015
.
- B. Mahar, C. Laslau, R. Yip and Y. Sun, IEEE Sens. J., 2007, 7, 266–284, DOI:10.1109/jsen.2006.886863
.
- H. Dai, Surf. Sci., 2002, 500, 218–241, DOI:10.1016/S0039-6028(01)01558-8
.
- K. Matzinger, Ph.D. thesis, Universität Freiburg, 2006, https://doc.rero.ch/record/6666/files/MatzingerK.pdf
.
- N. Gupta, S. Sharma, I. A. Mir and D. Kumar, J. Sci. Ind. Res., 2006, 65, 549–557 CAS
.
- B. Crawford, D. Esposito, V. Jain, D. Pelletier, C. Mavroidis, Y. J. Jung and A. Khanicheh, Paper 55, Mechanical Engineering Undergraduate Capstone Projects, Final Report, Northeastern University, 2007, http://iris.lib.neu.edu/cgi/viewcontent.cgi?article=1054%26context=mech_eng_capstone.
- A. Abbaspourrad, C. Verissimo, R. V. Gelamo, M. M. Silva, A. R. Vaz, F. P. M. Rouxinol, O. L. Alves and S. A. Moshkalev, in Smart Nanocomposites Research Compendium, ed. K. Levine, Nova Publishers, USA, 1st edn, 2013, vol. 1, ch. 2, pp. 13–20, https://www.novapublishers.com/catalog/product_info.php?products_id=25809 Search PubMed
.
- M. Ding, Ph.D. Thesis, University of Pittsburgh, 2013, http://d-scholarship.pitt.edu/19257/4/Mengning_Ding_ETD_2013.pdf
.
- Z. Chen and K. Saito, UCR Review Meeting, University of Kentucky, 2007, http://seca.doe.gov/publications/proceedings/05/UCR_HBCU/pdf/papers/Zhi_Chen.pdf Search PubMed
.
- M. D. Volder, D. Reynaerts and C. V. Hoof, Proceeding of the Conference on IEEE Sensors, 2010, pp. 2369–2372, http://mechanosynthesis.mit.edu/conferences/026_devolder_sensors10_bridgetempsensor.pdf Search PubMed
.
- A. Misra, Special section: Carbon Technology, Curr. Sci., 2014, 107, 419–429 CAS
.
- J. Wang and M. Musameh, Analyst, 2004, 129, 1–2, 10.1039/b313431h
.
- S. Peng and K. Cho, Nano Lett., 2003, 3, 513–517, DOI:10.1021/nl034064u
.
- S. Brahim, S. Colbern, R. Gump, A. Moser and L. Grigorian, Nanotechnology, 2009, 20, 1–7, DOI:10.1088/0957-4484/20/23/235502
.
- A. Abdelhalim, A. Abdellah, G. Scarpa and P. Lugli, Nanotechnology, 2014, 25, 1–10, DOI:10.1088/0957-4484/25/5/055208
.
- Z. Zanolli, R. Leghrib, A. Felten, J. J. Pireaux, E. Llobet and J. C. Charlier, ACS Nano, 2011, 5, 4592–4599, DOI:10.1021/nn200294h
.
- I. V. Anoshkin, A. G. Nasibulin, P. R. Mudimela, M. He, V. Ermolov and E. I. Kauppinen, Nano Res., 2013, 6, 77–86, DOI:10.1007/s12274-012-0282-6
.
- M. Ding, Y. Tang, P. Gou, M. J. Reber and A. Star, Adv. Mater., 2011, 23, 536–540, DOI:10.1002/adma.201003304
.
- D. R. Kauffman, D. C. Sorescu, D. P. Schofield, B. L. Allen, K. D. Jordan and A. Star, Nano Lett., 2010, 10, 958–963, DOI:10.1021/nl903888c
.
- T. Zhang, S. Mubeen, N. V. Myung and M. A. Deshusses, Nanotechnology., 2008, 19, 1–14, DOI:10.1088/0957-4484/19/33/332001
.
- J. M. Schnorr, D. V. Zwaag, J. J. Walish, Y. Weizmann and T. M. Swager, Adv. Funct. Mater., 2013, 23, 5285–5291, DOI:10.1002/adfm.201300131
.
- Y. Shang, X. He, Y. Li, L. Zhang, Z. Li, C. Ji, E. Shi, P. Li, K. Zhu, Q. Peng, C. Wang, X. Zhang, R. Wang, J. Wei, K. Wang, H. Zhu, D. Wu and A. Cao, Adv. Mater., 2012, 24, 2896–2900, DOI:10.1002/adma.201200576
.
- C. Oueiny, S. Berliozand and F. X. Perrin, Prog. Polym. Sci., 2014, 39, 707–748, DOI:10.1016/j.progpolymsci.2013.08.009
.
- P. Gajendran and R. Saraswathi, Pure Appl. Chem., 2008, 80, 2377–2395, DOI:10.1351/pac200880112377
.
- A. Firouzi, S. Sobri, F. M. Yasin and F. L. Ahmadun, Proceeding of the International Conference on Nanotechnology and Biosensors, Singapore, 2011, http://www.ipcbee.com/vol2/39-Y40002.pdf Search PubMed
.
- H. J. Salavagione, A. M. Diez-Pascual, E. Lazaro, S. Vera and M. A. Gomez-Fatoua, J. Mater. Chem. A, 2014, 2, 14289–14328, 10.1039/c4ta02159b
.
- A. C. Ferrari, F. Bonaccorso, V. Fal’ko, K. S. Novoselov, S. Roche, P. Bøggild, S. Borini, F. H. L. Koppens, V. Palermo, N. Pugno, J. A. Garrido, R. Sordan, A. Bianco, L. Ballerini, M. Prato, E. Lidorikis, J. Kivioja, C. Marinelli, T. Ryhänen, A. Morpurgo, J. N. Coleman, V. Nicolosi, L. Colombo, A. Fert, M. G. Hernandez, A. Bachtold, G. F. Schneider, F. Guinea, C. Dekker, M. Barbone, Z. Sun, C. Galiotis, A. N. Grigorenko, G. Konstantatos, A. Kis, M. Katsnelson, L. Vandersypen, A. Loiseau, V. Morandi, D. Neumaier, E. Treossi, V. Pellegrini, M. Polini, A. Tredicucci, G. M. Williams, B. H. Hong, J. H. Ahn, J. M. Kim, H. Zirath, B. J. V. Wees, H. V. D. Zant, L. Occhipinti, A. D. Matteo, I. A. Kinloch, T. Seyller, E. Quesnel, X. Feng, K. Teo, N. Rupesinghe, P. Hakonen, S. R. T. Neil, Q. Tannock, T. Löfwander and J. Kinaret, Nanoscale, 2015, 7, 4598–4810, 10.1039/c4nr01600a
.
- Z. Xu and C. Gao, Nat. Commun., 2011, 2, 571, DOI:10.1038/ncomms1583
.
- B. Weng, F. Xu and K. Lozano, J. Appl. Polym. Sci., 2015, 132(38), 42535, DOI:10.1002/app.42535
.
- D. P. Hansora, N. Shimpi and S. Mishra, JOM, 2015, 67(12), 2855–2868, DOI:10.1007/s11837-015-1522-5
.
- N. G. Shimpi, S. Mishra, D. P. Hansora and U. Savdekar, Indian Patent 3179/MUM/2013, 2013, http://ipindia.nic.in/ipr/patent/journal_archieve/journal_2013/pat_arch_102013/official_journal_25102013_part_i.pdf
.
- T. Sen, N. G. Shimpi and S. Mishra, Sens. Actuators, B, 2014, 190, 120–126, DOI:10.1016/j.snb.2013.07.091
.
- S. L. Patil, M. A. Chougule, S. Sen and V. B. Patil, Measurement, 2012, 45, 243–249, DOI:10.1016/j.measurement.2011.12.012
.
- D. S. Dhawale, R. R. Salunkhe, U. M. Patil, K. V. Gurav, A. M. More and C. D. Lokhande, Sens. Actuators, B, 2008, 134, 988–992, DOI:10.1016/j.snb.2008.07.003
.
- Z. Zanolli and J. C. Charlier, ACS Nano, 2012, 6, 10786–10791, DOI:10.1021/nn304111a
.
- E. Petryayeva and W. R. Algar, RSC Adv., 2015, 5, 22256–22282, 10.1039/c4ra15036h
.
- S. Mishra, N. G. Shimpi and T. Sen, J. Polym. Res., 2013, 49, 1–10, DOI:10.1007/s10965-012-0049-5
.
- B. Yeole, T. Sen, D. P. Hansora and S. Mishra, J. Appl. Polym. Sci., 2015, 132, 1–9, DOI:10.1002/app.42379
.
- C. S. Rout, M. Hegde and C. N. R. Rao, Sens. Actuators, B, 2008, 128, 488–493, DOI:10.1016/j.snb.2007.07.013
.
- Z. Sun, H. Yuan, Z. Liu, B. Han and X. Zhang, Adv. Mater., 2005, 17, 2993–2997, DOI:10.1002/adma.200501562
.
- G. Sberveglieri, Sens. Actuators, B, 1995, 23, 103–109, DOI:10.1016/0925-4005(94)01278-p
.
- L. A. Patil, M. D. Shinde, A. R. Bari, V. V. Deo, D. M. Patil and M. P. Kaushik, Sens. Actuators, B, 2011, 155, 174–182, DOI:10.1016/j.snb.2010.11.043
.
- V. K. Rana, S. Akhtar, S. Chatterjee, S. Mishra, R. P. Singh and C. S. Ha, J. Nanosci. Nanotechnol., 2014, 14, 2425–2435, DOI:10.1166/jnn.2014.8498
.
- V. K. Rana, M. C. Choi, J. Y. Kong, G. Y. Kim, M. J. Kim, S. H. Kim, S. Mishra, R. P. Singh and C. S. Ha, Macromol. Mater. Eng., 2011, 296(2), 131–140, DOI:10.1002/mame.201000307
.
- K. C. Persaud, Mater. Today, 2005, 8, 38–44, DOI:10.1016/s1369-7021(05)00793-5
.
- J. Janata and M. Josowicz, Nat. Mater., 2003, 2, 19–24, DOI:10.1038/nmat768
.
- L. Quercia, F. Loffredo, M. Bombace, A. D. Girolamo, D. Mauro and G. D. Francia, Proceeding of an International Conference on the Eurosensors, Italy, 2005, http://www.afs.enea.it/devito/Abstract_Eurosensors/ExtabsEUS05Comp.pdf Search PubMed
.
- S. Bagherbaigi, E. P. Córcoles and D. H. B. Wicaksono, Anal. Methods, 2014, 6, 7175, 10.1039/c4ay01071j
.
- H. Ago, N. Uehara, N. Yoshihara, M. Tsuji, M. Yumura, N. Tomonaga and T. Setoguchi, Carbon, 2006, 44, 2912–2918, DOI:10.1016/j.carbon.2006.05.049
.
- T. Yamada, T. Namai, K. Hata, D. N. Futaba, K. Mizuno, J. Fan, M. Yudasaka, M. Yumura and S. Iijima, Nat. Nanotechnol., 2006, 1, 131–136, DOI:10.1038/nnano.2006.95
.
- J. Huang and R. B. Kaner, Chem. Commun., 2006, 4, 367–376, 10.1039/b510956f
.
- P. Saini and V. Choudhary, J. Appl. Polym. Sci., 2013, 129, 2832–2839, DOI:10.1002/app.38994
.
- S. Iijima, Nature, 1991, 354, 56–58, DOI:10.1038/354056a0
.
- H. Cui, G. Eres, J. Y. Hawe, A. Puretkzy, M. Varela, D. B. Geohegan and D. H. Lowndes, Chem. Phys. Lett., 2003, 374, 222–228, DOI:10.1016/S0009-2614(03)00701-2
.
- J. M. Rosolen, L. A. Montoro, E. Y. Matsubara, M. S. Marchesin, L. F. do Nascimento and S. Tronto, in Activity Report LNLS 2006, Sci. Highlights, 2006, vol. 39, pp. 36–39, https://inis.iaea.org/search/searchsinglerecord.aspx?recordsFor=SingleRecord%26RN=39047187#.
- P. X. Hou, C. Liu and H. M. Cheng, Carbon, 2008, 46, 2003–2025, DOI:10.1016/j.carbon.2008.09.009
.
- R. Kar, S. G. Sarkar, C. B. Basak, A. Patsha, S. Dhara, C. Ghosh, D. Ramchandran, N. Chand, S. S. Chopade and D. S. Patil, Carbon, 2015, 94, 256–265, DOI:10.1016/j.carbon.2015.07.002
.
- J. Hong, J. Y. Han, H. Yoon, P. Joo, T. Lee, E. Seo, K. Char and B. S. Kim, Nanoscale, 2011, 3, 4515, 10.1039/c1nr10575b
.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453–5462, DOI:10.1021/nn401450s
.
- X. Liu, S. Cheng, H. Liu, S. Hu, D. Zhang and H. Ning, Sensors, 2012, 12, 9635–9665, DOI:10.3390/s120709635
.
- K. Jost, C. R. Perez, J. K. McDonough, V. Presser, M. Heon, G. Dion and Y. Gogotsi, Energy Environ. Sci., 2011, 4, 5060, 10.1039/c1ee02421c
.
- Y. P. Fu, X. Cai, H. W. Wu, Z. B. Lv, S. C. Hou, M. Peng, X. Yu and D. C. Zou, Adv. Mater., 2012, 24, 5713, DOI:10.1002/adma.201202930
.
- Y. Chae, J. T. Park, J. K. Koh, J. H. Kim and E. Kim, Mater. Sci. Eng., B, 2013, 178, 1117–1123, DOI:10.1016/j.mseb.2013.06.018
.
- T. Chen, L. Qiu, Z. Cai, F. Gong, Z. Yang, Z. Wang and H. Peng, Nano Lett., 2012, 12, 2568–2572, DOI:10.1021/nl300799d
.
- B. Adhikari and S. Majumdar, Prog. Polym. Sci., 2004, 29, 699–766, DOI:10.1016/j.progpolymsci.2004.03.002
.
- B. Liu, D. Tan, X. Wang, D. Chen and G. Shen, Small, 2013, 9, 1998–2004, DOI:10.1002/smll.201202586
.
- M. Stoppa and A. Chiolerio, Sensors, 2014, 14, 11957–11992, DOI:10.3390/s140711957
.
- H. Cheng, C. Hu, Y. Zhao and L. Qu, NPG Asia Mater., 2014, 6, 1–13, DOI:10.1038/am.2014.48
.
- A. Tsolis, W. G. Whittow, A. A. Alexandridis and J. C. Vardaxoglou, Electronics, 2014, 3, 314–338, DOI:10.3390/electronics3020314
.
- V. Kaushik, J. Lee, J. Hong, S. Lee, S. Lee, J. Seo, C. Mahata and T. Lee, Nanomaterials, 2015, 5, 1493–1531, DOI:10.3390/nano5031493
.
- E. Steven, W. R. Saleh, V. Lebedev, S. F. A. Acquah, V. Laukhin, R. G. Alamo and J. S. Brook, Nat. Commun., 2013, 4, 2435, DOI:10.1038/ncomms3435
.
- L. Liu, Y. Yu, C. Yan, K. Li and Z. Zheng, Nat. Commun., 2015, 6(7260), 1–9, DOI:10.1038/ncomms8260
.
- M. Krehel, M. Schmid, R. M. Rossi, L. F. Boesel, G. L. Bona and L. J. Scherer, Sensors, 2014, 14, 13088–13101, DOI:10.3390/s140713088
.
- L. L. Zhang, Z. Y. Wang and J. L. Volakis, IEEE Antennas and Wireless Propagation Letters, 2012, 11, 1690–1693, DOI:10.1109/lawp.2013.2239956
.
- I. Locher, M. Klemm, T. Kirstein and G. Troster, IEEE Trans. Adv. Packag., 2006, 29, 777–788, DOI:10.1109/tadvp.2006.884780
.
- E. K. Kaivanto, M. Berg, E. Salonen and P. de Maagt, IEEE Trans. Antennas Propag., 2011, 59, 4490–4496, DOI:10.1109/tap.2011.2165513
.
- G. Zhou, X. Mao and D. Juncker, Anal. Chem., 2012, 84, 7736–7743, DOI:10.1021/ac301082d
.
- N. G. Shimpi, D. P. Hansora, R. Yadav and S. Mishra, RSC Adv., 2015, 5(120), 99253–99269, 10.1039/c5ra16479f
.
Footnote |
| † The authors (Dr N. G. Shimpi and D. P. Hansora) have contributed equally as first author in this manuscript. |
| This journal is © The Royal Society of Chemistry 2015 |




