Facile synthesis of well-dispersed CeO2–CuOx composite hollow spheres with superior catalytic activity for CO oxidation†
Jian Zhangab,
Ming Gongb,
Yidan Caob and
Chang-An Wang*b
aSchool of Materials Science and Engineering, JingDeZhen Ceramic Institute, China
bState Key Laboratory of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, China. E-mail: wangca@mail.tsinghua.edu.cn
First published on 29th October 2015
Abstract
Well-dispersed CeO2–CuOx composite hollow spheres have been successfully synthesized through a facile reflux method using carbon spheres as sacrificial templates. The shells of the hollow spheres, ∼40 nm in thickness, consist of self-assembled 10–15 nm sized nanoparticles. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were employed to study the structural features of the CeO2–CuOx composite hollow spheres. X-ray photoelectron spectroscopy (XPS) confirmed that most of the copper element is distributed on the surface of the CeO2 shell support. The CeO2–CuOx composite hollow spheres exhibited enhanced catalytic activity for CO oxidation: complete CO conversion could be obtained at 112 °C. The excellent catalytic activity could be ascribed to the hollow structure, high specific surface area and the strong synergistic interaction between CeO2 and CuOx.
1. Introduction
Catalytic oxidation of CO has attracted considerable interest over the past several decades due to its potential applications in many fields, including automobile exhausts, fuel cells technology, carbon dioxide lasers and air purification in enclosed atmospheres.1–3 Owing to their excellent catalysis activity and low temperature oxidation, precious metal (platinum, gold, palladium, etc.) catalysts are widely studied.4–6 However, high prices and low reserves of noble metals have greatly limited their applications in practice. Therefore, more and more researchers have focused on preparation of “noble metal-free” catalysts. Recent studies7 reported that a ceria-based transition metal oxide catalyst exhibited enhanced CO oxidation activity due to the synergistic effect between transition metal oxides and ceria. Theoretical studies showed that the synergistic effect can lengthen or weaken the MO (M = transition metal) bond by sharing oxygen at the interface.Various kinds of composite oxide systems have been synthesized, including CeO2–CuOx,8,9 CeO2–ZnCo2O4,10 CeO2–MnO2,11 CeO2–ZnO,12 and CeO2–Fe2O3.13 Among them, the Ce–Cu binary oxide has attracted much attention because of its superior catalytic performance towards CO oxidation over other systems. Due to the significant advantages of low cost and high activity, CeO2–CuOx catalysts are the most promising “noble metal-free catalysts” in the future. Most of the traditional methods to synthesize Ce–Cu composite oxides were based on co-precipitation, sol–gel or combustion processes. Although these methods are simple and convenient, the resulting products tend to be unordered and could agglomerate easily in the process of catalytic reactions,4,14–16 which seriously aggravates the catalytic activity. Therefore, preparing the Ce–Cu composite oxide highly ordered and well-dispersed is an effective way to improve its performance and durability. For instance, HuizhiBao et al.17 successfully synthesized CeO2@Cu2O cubic-and octahedral-structured particles through liquid–solid interfacial reactions using Cu2O as a sacrificial template. The CeO2@Cu2O catalyst showed excellent activity which can attain 100% CO conversion at 170 °C. Dengsong Zhang et al.18 demonstrated the preparation of Cu doped CeO2 nanospheres using a solvothermal method, and 100% CO conversion was obtained at 210 °C. However, it still remains a great challenge for us to further improve the catalytic activity of Ce–Cu catalysts.
Among various nanomaterials, hollow sphere structures have attracted a great deal of attention because of their low density, high porosity and high specific surface area.19–21 It is well-known that porosity and specific surface area are important factors concerning catalyst activity. Therefore, over the past few years, many researchers have devoted themselves to developing various techniques to synthesize hollow spheres.22 Among all the preparation methods, the template-assisted method is the simplest and most widely used technique. In the process of preparation, the metal oxide nucleates and grows in situ on the surface of the template, which acts as the scaffold for the product, and thus the resulting product inherits the size and morphology of the template.20 However, to the best of our knowledge, very few works have been successful in synthesizing hierarchically well-dispersed CeO2–CuOx composite hollow spheres.
In this article, we report a novel self-assembled approach to prepare CeO2–CuOx composite hollow spheres in a reflux process, using colloid carbon spheres as sacrificial templates. The obtained CeO2–CuOx composite hollow spheres exhibit excellent catalytic activity for CO oxidation.
2. Experimental
Materials
Cerium(III) nitrate hexahydrate (Ce(NO3)3·6H2O), copper(II) nitratehydrate (Cu(NO3)2·3H2O), glucose (C6H12O6), hexamethylenetetramine (HMT) and absolute ethyl alcohol were used as starting materials. All the reagents are analytically pure and used without further purification. The deionized water used was purified by a Millipore Ultrapure water system.Processing
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 79.2) was passed through the reactor. Real-time concentrations of CO and CO2 of the resulting gases were analysed using an infrared gas analyser. The CeO2 hollow spheres and CeO2 particles were also tested for a better comparison.
79.2) was passed through the reactor. Real-time concentrations of CO and CO2 of the resulting gases were analysed using an infrared gas analyser. The CeO2 hollow spheres and CeO2 particles were also tested for a better comparison.3. Results and discussion
The CeO2–CuOx composite hollow spheres were prepared by employing carbon sphere as hard template. The as-prepared carbon spheres were monodispersed with a rather smooth surface, and the average diameter of the carbon spheres is about 400 nm (ESI Fig. S1†). Yadong Li et al.23 reported that carbon spheres had a large number of organic functional groups including –COOH, –CHO, and –OH on their surface, which is an indispensable qualification for coating. Owing to the negative charge of carbon spheres, certain metal ions can be adsorbed on their surface. With the help of a precipitant (HMT), the adsorbed metal ions will nucleate and turn into a nanooxide precipitate under reflux. After the process of self-assembly, uniform composite oxide shells can be achieved. Fig. 1a shows SEM images of CS/CeO2–CuOx microspheres, which indicate that all of the particles were still monodispersed and inherited the morphology of the carbon sphere templates, no uncoated particles were found. A magnified image (the inset of Fig. 1a) shows the detail of the rough surface of these microspheres, which can be attributed to the layer by layer self-assembly of grain. Additionally, the rough surface reveals the presence of a complete and homogenous shell on the carbon sphere template, which was further verified by the TEM images of the CS/CeO2–CuOx composite microsphere (Fig. 1b). The interface between the core and shell can be clearly observed and the thickness of the shell is about 40 nm. It is worth to mention that the thickness of the shell can be easily tuned by adjusting the concentration of the corresponding metal salts at the beginning of the reaction. Fig. 1c shows the SEM images of CeO2–CuOx composite hollow spheres obtained after the thermal treatment. The majority of obtained spheres were homogenous in size and spherical in shape, and only very few are broken down. The magnified micrographs (the inset of Fig. 1c) show that the surface of the microspheres was coarse, which is characteristic of mesoporous materials. Several spheres cracked (as the red arrows point out), demonstrating that the microspheres are hollow structures, which is further confirmed by TEM images (Fig. 1d). Moreover, the diameter of composite microspheres was found to decrease from 480 nm to 400 nm after calcination. This can be attributed to the sintering contraction of nanoparticles and further condensation of molecular precursors upon calcinations.24 Fig. 1d displays a TEM image of CeO2–CuOx composite hollow spheres where intact shells composed of nanoparticles can be clearly observed which was correspond the results of SEM. In addition, it can be observed the shell possess a porous structure with a macro-pore located at the center of nano-sphere. This hierarchical structural characteristic endows the sample with a high porosity and specific surface area. From the magnified TEM images (Fig. 1e), it is clear that the shell was made up of numerous of particles with particle sizes ranging from 10 nm to 15 nm. The lattices fringes in the high-resolution TEM (Fig. 1f) image show a spacing approximately 0.31 nm corresponding to the (111) planes of face-centered cubic fluorite structured CeO2. The corresponding SAED patterns (ESI Fig. S2†) suggest a single presence of CeO2 diffraction rings. Although the results seem strange, similar results were also reported in other ref. 25.In order to confirm the presence of both Ce species and Cu species in the shell of the composite microsphere, an energy dispersive spectroscopy (EDS) mapping analysis of the sample was conducted. The results are shown in Fig. 2b–e, where the yellow, red, green and pink regions refer to the elements of Ce, Cu, C and O, respectively. The results confirmed that both Ce and Cu species exist in the shell of the hollow spheres. The size of the C-enriched area was smaller than that of the area enriched in other elements, which indicated that there was a uniform coating layer on the surface of the carbon sphere. However, it should be mentioned that the copper species content was only 10 mol%, but from the spectroscopy, the content of Cu species seemed to be higher than that of Ce species, and there were two possible explanations for this phenomenon. Firstly, the TEM sample holder is made of copper, so it could influence the results. Secondly, the Cu species tend to be concentrated on the surface of the composite hollow sphere owing to its lower specific surface energy than that of the Ce species. The EDS patterns (Fig. 2f) also revealed that the composite hollow spheres contained both Ce and Cu species and that the mole ratio of Ce/Cu was 8.97/2.65.
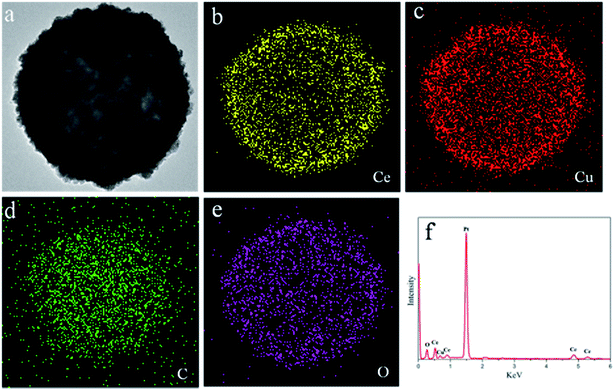 | ||
| Fig. 2 TEM (a) and element mapping (b–e) images of CeO2–CuOx@CS composite microsphere, and EDS energy spectrum of CeO2–CuOx hollow sphere (f). | ||
To investigate the phase composition of the samples, X-ray powder diffraction was performed and the results are shown in Fig. 3a. All the diffraction peaks can be indexed to the cubic fluorite structured CeO2 crystal (JCPDS 34-0394), which is in good agreement with the results of SAED and HRTEM. The broad nature of the patterns indicated that the CeO2–CuOx composite hollow spheres were composed of nano-crystals, which is consistent with the results of TEM analysis. No peak can be indexed to copper species. This can possibly be explained by the findings of previous researchers, who reported that the copper species cannot be detected by XRD when the contents is below 20 mol%.25
X-ray photoelectron spectroscopy was conducted to determine the valence states of Ce and Cu in the composite hollow sphere, and the results are shown in Fig. 3c and d, respectively. Six significant peaks (916.52, 907.22, 900.57, 898.02, 888.62 and 882.27 eV) clearly exist in the Ce 3d spectra, and they are all attributed to Ce(IV) feature. In the Cu 2p spectrum, both Cu2O (Cu 2p3/2 binding energy around 932.5 eV) and CuO (Cu 2p3/2 binding energy around 933.7 eV) features were observed.17 The Cu/Ce atomic ratio in the surface of the CeO2–CuOx composite hollow spheres were detected by XPS and displayed in Table 1. For CeO2–CuOx composite hollow spheres, the Cu/Ce atomic ratio on the surface is always higher than that of the real Cu/Ce molar ratio. It increases almost linearly with the corresponding real Cu/Ce mole ratio. Therefore, it can be concluded that the Cu species are highly concentrated on the surface of CeO2 hollow spheres. The Raman spectra of the samples prepared with various amounts of copper species are illustrated in Fig. 3b. The peak centered at 461 cm−1 is assigned to the symmetric breathing mode of oxygen atoms around cerium ions in the fluorite cerium oxide phase. It can be observed that with the increase of concentration of Cu species, the intensity of the peak decreases and the peak position shifts towards the low wavenumber area because of the strong interaction between the copper species and the CeO2 shell. It is worth to mention that the interaction between CeO2 and copper species can be divided into two aspects. As mentioned above (XPS spectra), copper species highly dispersed on the surface of CeO2 shell. The interaction between copper species and CeO2 contributes to the weakening of the metal–oxygen bond by sharing oxygen at the interface, which were the active sites for CO oxidation.9 Moreover, there are small number of copper species incorporates into the CeO2 lattice replace Ce4+. Due to the copper ion radius (0.072 nm) is smaller than that of Ce4+ (0.097 nm), so the Raman band shifts to low wavenumber with the increase of copper species.
| Sample | Pure CeO2 | 4%CuOx–CeO2 | 7%CuOx–CeO2 | 10%CuOx–CeO2 | 13%CuOx–CeO2 |
| Surface Cu mole percentage | 0 | 16.5% | 22.7% | 28.3% | 28.7% |
| (BET) surface area (m2 g−1) | 87.23 | 98.72 | 118.62 | 123.67 | 105.85 |
| T50 (temperature for 50% CO conversion) | 253 °C | 82 °C | 79 °C | 65 °C | 74 °C |
The FT-IR spectra of CS, CS/CeO2–CuOx composite microspheres, CeO2–CuOx (550 °C heat-treated) hollow spheres are show in Fig. 4a. The bands centered around 3462, 1706 and 1618 cm−1 correspond to –OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations, respectively. In addition, the bands in the range of 1000–1300 cm−1 contain C–OH stretching and OH bending vibrations.21 All of these functional groups can be attributed to the CS templates. It is interesting that after the coating of CeO2–CuOx composite precursor, the intensity of all these peaks did not decrease, but instead increase. This strange phenomenon can be explained as follows: the surface of the original carbon sphere template is hydrophilic, and Sa Li et al.22 reported that alkaline environment can help to increase the content of oxygen-containing groups, rendering the surface of the CS more hydrophilic. As the precipitant HMT provided an alkaline environment, the intensity of the feature peaks increased. After the thermal treatment at 550 °C, all the characteristic peaks of CS almost disappeared, signifying the complete removal of the template from the composite oxide.
C vibrations, respectively. In addition, the bands in the range of 1000–1300 cm−1 contain C–OH stretching and OH bending vibrations.21 All of these functional groups can be attributed to the CS templates. It is interesting that after the coating of CeO2–CuOx composite precursor, the intensity of all these peaks did not decrease, but instead increase. This strange phenomenon can be explained as follows: the surface of the original carbon sphere template is hydrophilic, and Sa Li et al.22 reported that alkaline environment can help to increase the content of oxygen-containing groups, rendering the surface of the CS more hydrophilic. As the precipitant HMT provided an alkaline environment, the intensity of the feature peaks increased. After the thermal treatment at 550 °C, all the characteristic peaks of CS almost disappeared, signifying the complete removal of the template from the composite oxide.
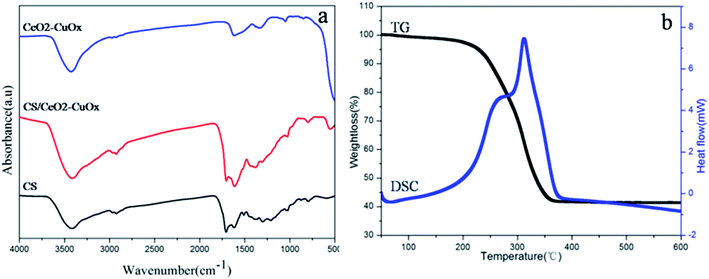 | ||
| Fig. 4 (a) FT-IR spectra of CS, CS/CeO2–CuOx nanospheres, CeO2–CuOx (550 °C heat-treated) and CeO2–CuOx (750 °C heat-treated) composite hollow spheres (b) TG-DSC results of CS/CeO2–CuOx nanospheres. | ||
Thermogravimetric (TG-DSC) analysis was employed to further investigate the CS/CeO2–CuOx composite particles (Fig. 4b). The weight loss can be divided into two stages. The first one occurs within a temperature range of 100 to 250 °C, about 5 wt%, which results from the removal of absorbed water and the residual solvent of composite particles. The second one, from 250 to 380 °C, is a significant weight loss of ∼55 wt%, which can be attributed to the decomposition and oxidation of the carbon sphere. Finally, 40 wt% CeO2–CuOx composite hollow spheres can be achieved from CS/CeO2–CuOx.
CO catalytic oxidation tests were conducted to investigate the catalytic activity of the CeO2–CuOx composite hollow spheres. Pure CeO2 hollow spheres and CeO2 nanoparticles were prepared and acted as control catalysts. T50, the temperature for 50% CO conversion is used to estimate the catalytic activity of the sample. The catalytic results for the above samples are shown in Fig. 5. As can be seen, the catalytic activity of the samples following such order of T50: 10%CuOx–CeO2 > 13%CuOx–CeO2 > 7%CuOx–CeO2 > 4%CuOx–CeO2 > CeO2 hollow sphere > CeO2 particle, and corresponding T50 values shown in Table 1. Furthermore, the T90 values of all CuOx–CeO2 composite hollow spheres are under 150 °C. For the samples D, E and F, the complete CO conversions attained at 112, 127 and 130 °C, respectively. While the light-off temperature of pure CeO2 hollow spheres and CeO2 nanoparticles reached as high as 150 °C, 90% CO conversion was obtained at 272 and 286 °C, respectively. Obviously, the CeO2–CuOx composite hollow spheres possess much higher catalytic activity than pure CeO2 hollow spheres and CeO2 nanoparticles. The 10%CuOx–CeO2 composite hollow spheres had the highest catalytic activity. The enhanced performance can be attributed to the strong synergistic interaction between copper species and cerium. From the results of XPS and XRD, it can be known that the copper species are well dispersed on the surface of CeO2, and the two phase interfaces act as chemisorption sites for CO.9 It is obviously found that, below 10 mol%, the increasing copper species contents can effectively increase the catalytic performance, which can be attributed to the increase of the active sites for CO catalytic oxidation. However, upon further increase of the amount of copper species, the bulk CuOx will form and can aggravate the catalytic activity.25 So in this experiment, the optimal content of copper species was found to be around 10 mol%. Next, a cycling test was performed to study the stability of 10%CuOx–CeO2 composite hollow spheres (Fig. 6). After five reaction cycles from 30 to 140 °C, the sample still maintains high catalytic activity with 100% CO conversion at 125 °C, which demonstrate the good cycle stability of CeO2–CuOx composite hollow spheres.
 | ||
| Fig. 5 CO conversion as a function of temperature for (A) CeO2 hollow sphere (B) CeO2 solid particle (C) 4% (D) 7% (E) 10% (F) 13% CuOx–CeO2 composite hollow spheres. | ||
The specific surface area also plays an important role in catalytic oxidation. It is well-known that a high surface area endows catalyst with better catalytic activity, due to the fact that higher surface areas can provide more active sites. The N2 adsorption–desorption isotherms of CeO2 and CeO2–CuOx composite hollow spheres are illustrated in Fig. S3.† All the samples exhibited type IV isotherms with H2 type hysteresis loops, indicating typical mesoporous structures. The specific surface areas of all the samples are summarized in Table 1. As can be seen, the specific surface area of the CeO2–CuOx composite hollow spheres will be increased along with the increase of copper species contents at first and then decreased when the content of copper species is more than 10 mol%. There is no doubt that copper species are the active sites for CO oxidation, so the increase of specific surface area and copper species means the increase of reactivity sites when the contents of copper species stay below 10 mol%. Therefore, corresponding T50 values decrease. However, the T50 values actually decrease when the content of copper species is more than 10 mol%. It is related to the formation of bulk CuOx as mentioned above, since the activity of bulk CuOx is lower than highly dispersed CuOx.9
4. Conclusions
In summary, we developed a simple self-assembly method to prepare CeO2–CuOx composite hollow spheres using colloid carbon spheres as sacrificial templates. The hollow structures can be obtained after a simple thermal treatment in an air environment. Copper species are highly dispersed on the surface of CeO2 shell. Due to the high specific surface area and the strong synergistic interaction between CeO2 and CuOx in the CeO2–CuOx composite hollow spheres, the samples display excellent catalytic activity for CO oxidation. 10 mol% Cu species is the optimal content in the composite oxide, which can attain 100% CO conversion at 112 °C. In particular, this method performed admirably and can be easily extended to synthesize other composite hollow spheres.Acknowledgements
The authors would like to thank the financial support from the National Science Foundation of China (NSFC-No. 51221291 and 51172119).Notes and references
- D. Gamarra, C. Belver, M. Fernández-García and A. Martínez-Arias, J. Am. Chem. Soc., 2007, 129, 12064–12065 CrossRef CAS PubMed.
- P. G. Harrison, I. K. Ball, W. Azelee, W. Daniell and D. Goldfarb, Chem. Mater., 2000, 12, 3715–3725 CrossRef CAS.
- X. Zheng, X. Zhang and Z. Fang, Catal. Commun., 2006, 701–704 CrossRef CAS.
- P. Bera, A. Gayen and M. S. Hegde, J. Phys. Chem. B, 2003, 107, 6122–6130 CrossRef CAS.
- S. Carrettin, P. Concepción, A. Corma, J. M. López Nieto and V. F. Puntes, Angew. Chem., Int. Ed., 2004, 43, 2538–2540 CrossRef CAS PubMed.
- X. Wang, D. Liu, S. Song and H. Zhang, J. Am. Chem. Soc., 2013, 135, 15864–15872 CrossRef CAS PubMed.
- V. Shapovalov and H. Metiu, J. Catal., 2007, 245, 205–214 CrossRef CAS.
- J. Qin, J. Lu, M. Cao and C. Hu, Nanoscale, 2010, 2, 2739–2743 RSC.
- A. Jia, S. Jiang, J. Lu and M. Luo, J. Phys. Chem. C, 2010, 114, 21605–21610 CAS.
- F. Wang, X. Wang, D. Liu, J. Zhen, J. Li, Y. Wang and H. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 22216–22223 CAS.
- G. Chen, F. Rosei and D. Ma, Adv. Funct. Mater., 2012, 22, 3914–3920 CrossRef CAS.
- Q. Xie, Y. Zhao, H. Guo, A. Lu, X. Zhang, L. Wang, M. Chen and D. Peng, ACS Appl. Mater. Interface, 2013, 6, 421–428 CrossRef PubMed.
- G. Avgouropoulos and T. Ioannides, Catal. Lett., 2007, 116, 15–22 CrossRef CAS.
- R. Prasad and G. Rattan, Bull. Chem. React. Eng. Catal., 2010, 5, 7–30 CAS.
- L. Camara, A. Kubacka, Z. Schay and Z. Koppány, J. Power Sources, 2011, 9, 4364–4369 CrossRef.
- S. H. Zeng, Y. Liu and Y. Q. Wang, Catal. Lett., 2007, 117, 119–125 CrossRef CAS.
- H. Bao, Z. Zhang, Q. Hua and W. Huang, Langmuir, 2014, 30, 6427–6436 CrossRef CAS PubMed.
- D. Zhang, Y. Qian, L. Shi, H. Mai, R. Gao, J. Zhang, W. Yu and W. Cao, Catal. Commun., 2012, 26, 164–168 CrossRef CAS.
- J. Wang, N. Yang and H. Tang, Angew. Chem., Int. Ed., 2013, 6545–6548 CrossRef.
- M. Agrawal, S. Gupta, A. Pich, N. E. Zafeiropoulos and M. Stamm, Chem. Mater., 2009, 21, 5343–5348 CrossRef CAS.
- M. Chen, C. Ye, S. Zhou and L. Wu, Adv. Mater., 2013, 25, 5343–5351 CrossRef CAS PubMed.
- C. Wang, S. Li and L. An, Chem. Commun., 2013, 49, 7427–7429 RSC.
- X. Sun and Y. Li, Angew. Chem., Int. Ed., 2004, 43, 597–601 CrossRef PubMed.
- D. Wang and F. Caruso, Chem. Mater., 2002, 14, 1909–1913 CrossRef CAS.
- M. Luo, Y. Song, J. Lu, X. Wang and Z. Pu, J. Phys. Chem. B, 2007, 111, 12686–12692 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16410a |
| This journal is © The Royal Society of Chemistry 2015 |



