Dual responsive macroemulsion stabilized by Y-shaped amphiphilic AB2 miktoarm star copolymers†
Heng Lia,
Duanguang Yanga,
Yong Gao*ab,
Huaming Liab and
Jianxiong Xu*ac
aCollege of Chemistry, Xiangtan University, Xiangtan, Hunan Province 411105, China. E-mail: gydx.1027@163.com
bKey Laboratory of Polymeric Materials & Application Technology of Hunan Province, Key Laboratory of Advanced Functional Polymeric Materials of College of Hunan Province, Key Lab of Environment Friendly Chemistry and Application in Ministry of Education, Xiangtan, Hunan Province 411105, China
cHunan Key Laboratory of Green Packaging & Application of Biological Nanotechnology, Hunan University of Technology, Zhuzhou, Hunan Province 412007, China
First published on 30th October 2015
Abstract
Dual responsive macroemulsions stabilized by Y-shaped amphiphilic AB2 miktoarm star polymeric emulsifiers were presented in this study. First, a amphiphilic Y-shaped AB2 miktoarm star polymer composed of poly(N,N-dimethylaminoethylmethacrylate) (PDMAEMA) and polystyrene (PS) arms was synthesized by sequential reversible addition–fragmentation chain transfer (RAFT) polymerization of styrene monomer and atom transfer radical polymerization (ATRP) of N,N-dimethylaminoethyl methacrylate (DMAEMA) monomer. The structure and the molecular weight as well as the molecular weight distribution were carefully characterized by 1H NMR and GPC, respectively. The obtained PS–(PDMAEMA)2 miktoarm star polymers were then applied as polymer emulsifiers for both o/w and w/o macroemulsions formation, and stabilized macroemulsions could be produced at a lower emulsifier content. Meanwhile, the emulsifying performance of PS–(PDMAEMA)2 miktoarm star polymer and stimulus-response of the macroemulsion were also investigated. The PS–(PDMAEMA)2 stabilized o/w macroemulsion showed pH-induced demulsification and temperature-induced phase inversion. However, the inversion of the PS–(PDMAEMA)2 emulsifier at the oil–water interface could not be spontaneously accomplished. Furthermore, successful phase inversion was only smoothly realized for those emulsions with pH 7 water in the presence of a modulate stirring.
1. Introduction
Emulsions are a class of disperse systems consisting of two immiscible liquids,1 where disperse phase droplets are dispersed in a continuous phase medium in the presence of emulsifiers. Emulsions, not including Pickering emulsion,2 can be divided into three types: a kinetically stable macroemulsion, thermodynamically stable microemulsion, and an “approaching thermodynamically stable” nanoemulsion3 according to the droplet size; the corresponding droplet size ranges are 0.1–5 μm, 5–50 nm and 20–100 nm, respectively.4 Because of their high stability, microemulsions and nanoemulsions have huge amounts of applications in food technology, personal care and cosmetics, and drug delivery as well as materials synthesis.5–9 However, preparation of a microemulsion is not easy due to a lack in general theory for predicting the microemulsion structure in a particular system containing oil, water, and surfactant.10 From this point of view, preparation of kinetically stable macroemulsions is relatively easy because they can be obtained by simply dispersing oil and water phases in the presence of emulsifiers without a co-stabilizer requirement. Furthermore, a large number of surfactants with a lower concentration and a wider range of volume ratio of the two phases are suitable for macroemulsion formation.11,12Both low molecular weight surfactants and polymeric surfactants can be utilized to stabilize macroemulsions.13 Additionally, inorganic solid particles or polymer soft particles with suitable wettability can also be used as macroemulsion stabilizers, and these particle-stabilized emulsions are specially termed as “Pickering emulsions”.2,14–18 Compared to traditional low molecular weight surfactants, amphiphilic block copolymers usually have a very low critical micelle concentration and a low diffusion coefficient.19 As a result, a lower polymeric surfactant content is required for emulsion formation. So far, many amphiphilic block polymers with different constitutes have been applied for macroemulsions in past decades.20–24 However, to the best of our knowledge, polymeric surfactants for macroemulsions were mainly concentrated on linear amphiphilic block copolymers, and less attention was paid to amphiphilic miktoarm star polymers.25
Miktoarm star polymers, also called asymmetric star polymers or hetero-arm star polymers, refer to polymers that contain a central core connected by a number of various types of polymer arms.26,27 During the past decades, a wealth of miktoarm star polymers with varied arm constitutions have been synthesized by virtue of the great progress in polymer synthesis methodology.28–34 Y-shaped AB2 miktoarm polymers, as the simplest miktoarm star polymers, have received considerable attention within the past years. Synthesis strategies for Y-shaped AB2 miktoarm polymers include atom transfer radical polymerization (ATRP),35,36 opening polymerization (ROP),37–39 and “click” reaction40 as well as versatile combinations of different polymerization methods, including ATRP/ROP,41,42 reversible addition–fragmentation chain transfer (RAFT) polymerization/ROP,43 ROP/click,44 ATRP/ROP/click,45 living cationic polymerization/ROP, and anionic polymerization/ROP combination.46,47 Meanwhile, self-assembly of Y-shaped AB2 miktoarm polymers in solution also has been investigated in detail. Experimental results showed that self-assembly in solution of Y-shaped AB2 miktoarm polymers is different from that of their linear counterparts.37–39,41 Distinctions in self-assembly behaviors suggested that Y-shaped AB2 miktoarm polymers should have rather different interfacial properties from their linear counterparts. However, as far as we are concerned, there have been limited reports on a macroemulsion stabilized by Y-shaped AB2 miktoarm polymers to date.25
Herein, we presented a dual responsive macroemulsion using a well-defined AB2 miktoarm star polymer composed of poly(N,N-dimethylaminoethyl methacrylate) (PDMAEMA) and polystyrene (PS) arms as polymeric emulsifiers in this study. PS–(PDMAEMA)2 was synthesized by sequential reversible addition–fragmentation chain transfer (RAFT) polymerization of styrene monomer and atom transfer radical polymerization (ATRP) of DMAEMA monomer. The structure and the molecular weight as well as the molecular weight distribution of PS–(PDMAEMA)2 were carefully characterized by GPC and 1H NMR, respectively. The emulsifying performance of emulsifier and the stimuli-responses of the formed macroemulsion were investigated in detail.
2. Experimental
2.1 Materials
Trimethylolpropane was obtained from Aldrich and used without further purification. N,N-Dimethylaminoethyl methacrylate (DMAEMA) was purified by passing it through a dried basic alumina column and distilling over CaH2 under reduced pressure prior to polymerization. Styrene was purified by reduced pressure distillation. 2-(Ethylthiocarbonothioylthio)-2-methylpropanoate (EMP) was synthesized according to procedures described in the literature.48 2-Bromoisobutyryl bromide (BrIB), N,N,N′,N′′,N′′-pentamethyldiethylenetriamine (PMDETA), 1,3-dicyclohexylcarbodiimide (DCC), and 4-(dimethylamino)pyridine (DMAP) were purchased from Aldrich and used as received. Azobisisobutyronitrile (AIBN) was recrystallized twice from ethanol prior to use.2.2 Synthesis of trifunctional core
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent, affording a pale yellow oil. Yield: 5.34 g (78.5%). 1H NMR (400 MHz, CDCl3, δ, ppm): 4.14 (s, 2H, OCH2), 3.59 (s, 4H, CH2OH), 3.33–3.27 (q, 2H, SCH2), 1.71 (s, 6H, CCH3), 1.36–1.30 (q, 2H, CCH2), 1.32–1.25 (t, 3H, SCH2CH3), 0.88–0.84 (t, 3H, CH2CH3).
1) as an eluent, affording a pale yellow oil. Yield: 5.34 g (78.5%). 1H NMR (400 MHz, CDCl3, δ, ppm): 4.14 (s, 2H, OCH2), 3.59 (s, 4H, CH2OH), 3.33–3.27 (q, 2H, SCH2), 1.71 (s, 6H, CCH3), 1.36–1.30 (q, 2H, CCH2), 1.32–1.25 (t, 3H, SCH2CH3), 0.88–0.84 (t, 3H, CH2CH3).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent, affording a pale yellow oil. Yield: 7.16 g (74.8%). 1H NMR (400 MHz, CDCl3, δ, ppm), 4.10 (s, 4H, OCH2), 4.04 (s, 2H, OCH2), 3.30–3.25 (q, 2H, SCH2), 1.94 (s, 12H, C(Br)CH3), 1.70 (s, 6H, CCH3), 1.60–1.54 (t, 2H, CCH2), 1.34–1.30 (q, 3H, SCH2CH3), 0.94–0.91 (t, 3H, CCH2CH3).
1) as an eluent, affording a pale yellow oil. Yield: 7.16 g (74.8%). 1H NMR (400 MHz, CDCl3, δ, ppm), 4.10 (s, 4H, OCH2), 4.04 (s, 2H, OCH2), 3.30–3.25 (q, 2H, SCH2), 1.94 (s, 12H, C(Br)CH3), 1.70 (s, 6H, CCH3), 1.60–1.54 (t, 2H, CCH2), 1.34–1.30 (q, 3H, SCH2CH3), 0.94–0.91 (t, 3H, CCH2CH3).2.3 Synthesis of PS–Br2 ATRP macroinitiator by RAFT polymerization
PS–Br2 macroinitiator for ATRP was obtained by RAFT polymerization of St employing EPBP as a RAFT agent. In a typical reaction, St (1.248 g, 12 mmol), EPBP (0.128 g, 0.2 mmol) and AIBN (10.93 mg, 0.067 mmol) were charged into a round bottomed flask. The flask was degassed by three freeze–pump–thaw cycles and then immersed in oil bath at 75 °C for polymerization under N2 atmosphere. After 5 h of polymerization, the reaction was quenched by diluting with THF. After multiple operations involving precipitation in methanol, filtration, and washing, the product was dried under vacuum at room temperature.2.4 Synthesis of Y-shaped PS–(PDMAEMA)2 miktoarm star copolymer via ATRP
PS–(PDMAEMA)2 miktoarm star copolymer was synthesized by ATRP of DMAEMA in THF at 60 °C using PS–Br2 and CuBr/PMDETA as macroinitiator and catalyst, respectively. In a typical reaction, 0.4 g of PS macroinitiator, CuBr (28.7 mg, 0.2 mmol), PMDETA (0.041 mL, 0.2 mmol), DMAEMA (2.51 g, 16 mmol) and 3 mL of fresh THF were charged into a round bottomed flask. The flask was degassed by three freeze–pump–thaw cycles and then immersed in an oil bath at 60 °C for polymerization under N2. After 4 h of polymerization, the reaction was stopped by diluting with THF and then passed through a short basic alumina column to remove the metal salt. The solution was concentrated by evaporation, and the concentrated mixture was dropped into a large amount of hexane, producing a white precipitation. The precipitate was filtered, and washed with hexane. These precipitation, filtration and washing operations were repeated for three times, and the purified product was dried under vacuum at room temperature.2.5 Generation of toluene/water (o/w) and water/toluene (w/o) emulsions stabilized by PS–(PDMAEMA)2 miktoarm star copolymers
Typical preparation procedures of o/w and w/o emulsions were as follows: an equal volume of PS–(PDMAEMA)2 miktoarm star copolymer toluene solution (5 mg mL−1) and acidic water (pH 5) were charged in a 10 mL of glass vial at room temperature. Emulsification was performed using a XHF-D high speed disperser (Ningbo Scientz Biotechnology Co., Ltd, China) at a stirring rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. During homogenization, the glass vial was immersed in an ice bath to remove heat formed by this process. The emulsion droplets were observed using an optical microscope. A few drops of the diluted emulsions were placed on a glass slide and viewed. The type of emulsion was determined by conductivity using a Jenway 4510 conductivity meter.
000 rpm for 1 min. During homogenization, the glass vial was immersed in an ice bath to remove heat formed by this process. The emulsion droplets were observed using an optical microscope. A few drops of the diluted emulsions were placed on a glass slide and viewed. The type of emulsion was determined by conductivity using a Jenway 4510 conductivity meter.
2.6 pH- and thermo-response of o/w emulsion
The pH-response of an o/w emulsion was performed by adding a known volume of 1 mol L−1 NaOH aqueous solution into an emulsion to adjust the water phase pH to about 9.0. For the broken emulsion, a known volume of 1 mol L−1 HCl aqueous solution was added into the water to adjust the water phase pH to 5.0. Re-emulsification was performed by homogenization at a stirring rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min. The thermo-response of an o/w emulsion was performed by heating the formed o/w emulsion in a water bath at 55 °C alone or with a gentle shaking by hand or with moderate magnetic stirring. All pH values of the water phase were measured by pH meter.
000 rpm for 1 min. The thermo-response of an o/w emulsion was performed by heating the formed o/w emulsion in a water bath at 55 °C alone or with a gentle shaking by hand or with moderate magnetic stirring. All pH values of the water phase were measured by pH meter.
2.7 Characterization tests
1H NMR spectra were recorded with a Bruker AV-400 NMR spectrometer. Molecular weights and polydispersity indexes (PDI) of polymers were determined by GPC measurements, which were performed on a Waters 1515 GPC equipped with a Waters 2414 differential refractive index detector in DMF at 80 °C with a row rate of 1.0 mL min−1. Interfacial tension measurements were measured by the Wilhelmy plate method, and the experiments were performed with a Kruss tensiometer K20 equipped with a Wilhelmy slide. Optical micrographs (OM) were collected with an optical microscope (Leica, DM 4500P). A drop of the diluted emulsion was placed on a microscope slide and pictures were taken randomly from different spots of the same sample. The average size of droplets was analyzed by a MALVERN Zetasizer Nano ZS instrument. Confocal scanning laser microscopy (CSLM, Olympus FV1000 laser confocal microscope, Japan) was used to observe the primary o/w emulsion and the formed w/o emulsion by a thermo-induced inversion process. The o/w emulsion employed for a CLSM image was stabilized by 0.12 wt% of PS32–(PDMAEMA121)2 miktoarm star copolymers. Prior to homogenization, a hydrophilic fluorescence probe of Rhodamine B (RhB) and a hydrophobic fluorescence probe of allyl-(7-nitro-benzo[1,2,5]-oxadiazol-4-yl)-amine (NBDAA) were dissolved in water and toluene, respectively. The o/w emulsion was heated in a water bath at 55 °C for 5 min with moderate stirring.3. Results and discussion
3.1 Synthesis of Y-shaped PS–(PDMAEMA)2 miktoarm star copolymer
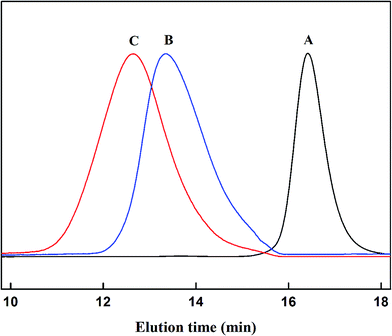
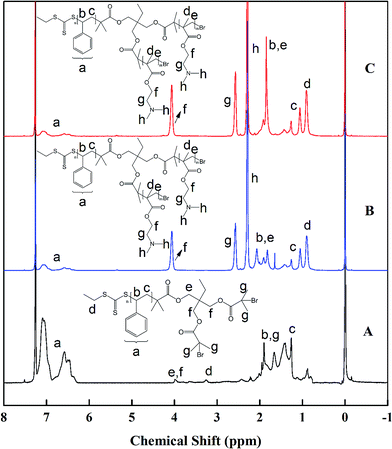
In this study, Y-shaped PS–(PDMAEMA)2 miktoarm star copolymer was synthesized using “core first” strategy. The overall synthesis route was shown in Scheme 1. As illustrated in Scheme 1, a trifunctional core of EPBP containing two ATRP initiator groups and RAFT group was first synthesized by two successive chemical processes involving the synthesis of BEMP by an esterification reaction of trimethylolpropane with EMP, and the subsequent acylation reaction between BEMP and 2-bromoisobutyryl bromide. Successful synthesis of the titled EPBP was verified by 1H NMR studies. Fig. 1 shows the 1H NMR spectra of EMP, BEMP, and EPBP. As displayed in Fig. 1A, the characteristic signals at δ = 3.31, 1.74, and 1.33 ppm were assigned to the protons of –SCH2, –CCH3 and –SCH2CH3 in EMP, which agrees with earlier reports.48 The 1H NMR spectrum of BEMP is displayed in Fig. 1B. Compared with the 1H NMR spectrum of EMP, four groups of new resonances at δ = 4.14, 3.59, 1.31, and 0.86 ppm appeared in addition to the characteristic signals of EMP. These newly formed signals were assigned to the protons of –CH2 next to an ester group, –CH2OH, and –CH2 as well as –CH3, respectively. Based on 1H NMR analysis, all peak integrals were consistent with the target compound. The appearance of these new resonance peaks and the consistence of peak integrals with the target compound were an indication of the successful esterification reaction between EMP and trimethylolpropane. The trifunctional core of EPBP was formed by further reaction of the produced BEMP with 2-bromoisobutyryl bromide. A new resonance peak at δ = 4.10 ppm was observed in the 1H NMR spectrum of the resultant EPBP, as shown in Fig. 1C. This new resonance peak was assigned to the protons of –CH2 next to –COOC(CH3)2Br. At the same time, another resonance peak at δ = 3.59 ppm in Fig. 1B, corresponding to the protons of –CH2OH, completely disappeared after the acylation reaction. This suggested that the –CH2OH groups in BEMP had been converted to –COOC(CH3)2Br. 1H NMR analysis also confirmed that peak integrals were consistent with the target EPBP.The purified trifunctional core of EPBP was then applied for the bulk RAFT polymerization of St, and the polymerization was carried out at 75 °C using AIBN as an initiator. After 5 h of polymerization, the reaction was stopped. The purified polymer was analyzed by GPC and 1H NMR. The GPC trace of PS polymer (denoted as PS–Br2) was shown in Fig. 2A. A symmetrical monomodal peak was an indication of a controlled RAFT polymerization of St mediated by EPBP using the described reaction conditions. GPC analysis indicated the Mn of PS–Br2 was 4100 g mol−1 with PDI of 1.13.
Fig. 3A shows the 1H NMR spectrum of PS–Br2, in which the respective resonances, including the bromine end groups, were clearly assigned. Mn of PS–Br2 was calculated to be 3900 g mol−1 according to the 1H NMR integral area ratio of a peak at δ = 7.23–6.50 ppm to that at δ = 3.28 ppm. Evidently, Mn calculated from 1H NMR analysis is approximately consistent with that from GPC result.
Purified PS–Br2 was then used as macroinitiator to initiate the ATRP of DMAEMA monomer to synthesize Y-shaped AB2 miktoarm star copolymers with different PDMAEMA block lengths. ATRP of DMAEMA was carried out in THF at 60 °C. After polymerization, the polymer was purified and then analyzed by GPC. By comparison with PS–Br2, GPC traces of PS–(PDMAEMA)2 presented a clear shift to a higher molecular weight region, as shown in Fig. 2B and C. The resulting symmetrical GPC monomodal peak in GPC curves revealed a controlled ATRP process of DMAEMA initiated by PS–Br2. GPC analysis indicated that the values of the Mn of the PS–(PDMAEMA)2 product were 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 42
000 and 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively, corresponding to 1.28 and 1.25 of PDI. According to GPC analysis, the number average polymerization degree of a PS block was 32, and a PDMAEMA block was 80 and 121, respectively. As a result, the as-synthesized two Y-shaped PS–(PDMAEMA)2 miktoarm star copolymers were thus denoted as PS32–(PDMAEMA80)2 and PS32–(PDMAEMA121)2. The 1H NMR spectra of PS–(PDMAEMA)2 with different PDMAEMA block lengths were displayed in Fig. 3B and C. Compared with Fig. 3A, several groups of new resonances at δ = 4.06, 2.57, 2.29, 1.84, and 0.91 ppm appeared. These resonances should be assigned to the protons of –OCH2, –OCH2CH3, –N(CH3)2, –CCH2 and –CCH3 in the PDMAEMA arms, respectively.49 1H NMR analysis indicated the values of Mn of the synthesized two PS–(PDMAEMA)2 samples are 21
000 g mol−1, respectively, corresponding to 1.28 and 1.25 of PDI. According to GPC analysis, the number average polymerization degree of a PS block was 32, and a PDMAEMA block was 80 and 121, respectively. As a result, the as-synthesized two Y-shaped PS–(PDMAEMA)2 miktoarm star copolymers were thus denoted as PS32–(PDMAEMA80)2 and PS32–(PDMAEMA121)2. The 1H NMR spectra of PS–(PDMAEMA)2 with different PDMAEMA block lengths were displayed in Fig. 3B and C. Compared with Fig. 3A, several groups of new resonances at δ = 4.06, 2.57, 2.29, 1.84, and 0.91 ppm appeared. These resonances should be assigned to the protons of –OCH2, –OCH2CH3, –N(CH3)2, –CCH2 and –CCH3 in the PDMAEMA arms, respectively.49 1H NMR analysis indicated the values of Mn of the synthesized two PS–(PDMAEMA)2 samples are 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 28
000 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively, which are smaller than those obtained from GPC determination.
000 g mol−1, respectively, which are smaller than those obtained from GPC determination.
3.2 PS–(PDMAEMA)2 miktoarm star copolymer stabilized o/w and w/o macroemulsions
Y-shaped PS–(PDMAEMA)2 miktoarm star copolymer has a hydrophobic PS arm and two hydrophilic PDMAEMA arms. This unique chain architecture might allow this unsymmetrical amphiphilic miktoarm star copolymer to have different emulsifying properties from those of its traditional amphiphilic linear counterpart. In order to verify this, we attempted to prepare emulsion using synthesized PS–(PDMAEMA)2 miktoarm star copolymers as emulsifiers, where toluene was used as the oil phase by virtue of its good solubility to both PS and PDMAEMA arms. Although PDMAEMA polymer can be well dissolved in some common organic solvents, such as THF, toluene etc., PDMAEMA is capable of being preferentially solvated by a water phase, especially acidic water, which allows PS–(PDMAEMA)2 chains to migrate from the initial toluene bulk phase to the water–oil interface during homogenization. At the water–oil interface, the PDMEMA arms were wetted by the water phase, while the PS block remained in the bulk toluene. PS–(PDMAEMA)2 miktoarm star copolymers absorbed at the water–toluene interface will stabilize the dispersed droplets and behave like surfactants. Fig. 4 shows representative photographs of the generated macroemulsions which were generated under different conditions. Our extensive experiments showed that PS32–(PDMAEMA121)2 showed enhanced emulsifying performances with the reduction in water pH. As shown in Fig. 4A and B, the oil phase was completely emulsified by pH 5 water with a fixed water/toluene volume ratio of 1 and a fixed PS32–(PDMAEMA121)2 content, whereas three layers, including the upper oil layer, the middle emulsion layer, and the under water layer, were produced at higher pH values such as 6 and 7, as indicated in Fig. 4C and D. After 24 h of standing at room temperature, the emulsion thickness was 55%, 30%, and 20% relative to the total liquid layer thickness, respectively (Fig. 4B, C and D). Emulsion could not be formed when more basic water, such as pH 9 water, was employed (Fig. 4E). Optical microscope photos of emulsions stabilized by different PS32–(PDMAEMA121)2 contents were also displayed in Fig. 4. The average size of emulsion droplets was about 3.6 μm with 0.233 of PDI (Fig. 4I), and 3.4 μm with 0.7 of PDI (Fig. 4J), corresponding to 0.12 wt% and 0.06 wt% of PS32–(PDMAEMA121)2 content, respectively. Increasing PS32–(PDMAEMA121)2 content was somewhat favorable to the reduction in the polydispersity instead of the average size of emulsion droplets. Meanwhile, PS–(PDMAEMA)2 showed a decreased emulsifying performance when the PDMAEMA block length was reduced. For example, the least required PS32–(PDMAEMA80)2 content was 0.10 wt% when toluene oil was completely emulsified by an equal volume of acidic water (pH 5), whereas the average size and the polydispersity of emulsion droplets were approximately the same as those of the emulsion droplets stabilized by 0.06 wt% of PS32–(PDMAEMA121)2 (S1, ESI†).The emulsifying performance of Y-shaped PS–(PDMAEMA)2 miktoarm star copolymer was also compared with that of its linear counterpart. For this purpose, a linear di-block copolymer of PS30-b-PDMAEMA259, possessing a Mn approximately the same as that of PS32–(PDMAEMA121)2, was synthesized by two successive ATRP processes using ethyl-2-bromoisobutanoate and CuBr/PMDETA as initiator and catalyst, respectively. The detailed synthesis and GPC characterizations are displayed in ESI (S2, ESI†). The number average polymerization degrees of PS and PDMAEMA block were 30 and 259, respectively, which was determined by GPC analysis. The obtained PS30-b-PDMAEMA259 was also used as an emulsifier for macroemulsion formation under the same conditions. Experimental observation revealed that toluene oil could not be emulsified completely by an equal volume of acidic water (pH 5) at 0.06 wt% of PS30-b-PDMAEMA259 content (Fig. 4G). Most of the toluene was separated from the emulsion after 24 h of standing. The emulsion layer thickness was about 25% relative to the total liquid layer. Only a thin emulsion layer was obtained even when the volume ratio of toluene to water was decreased to 0.25, and a separated oil layer could still be observed (Fig. 4H). The required PS30-b-PDMAEMA259 content was 0.12 wt% for the complete emulsification of toluene oil by an equal volume of pH 5 water (Fig. 4F). The average size of emulsion droplets was almost 3 μm (Fig. 4K). Obviously, the emulsion performance of PS32–(PDMAEMA121)2 miktoarm star copolymer was higher than that of its linear counterpart of PS30-b-PDMAEMA259. The differences of self assembly in solution of Y-shaped AB2 miktoarm star copolymers from their linear counterparts have been explained by several research groups.41,50,51 A Y-shaped AB2 miktoarm star copolymer with two hydrophilic arms was more inclined to self-assemble into a spherical micelle with a more compact core than its linear counterpart. Due to crowding of polymer branches at the junction point, each copolymer chain was required to occupy a larger surface area at the core–corona interface to accommodate two hydrophilic arms in an equilibrium conformation. Therefore, the micelles formed by self assembly of Y-shaped AB2 miktoarm star copolymers have a lower aggregation number and a smaller core size compared with their linear counterpart.41 With the present system, o/w emulsions stabilized by 0.06 wt% of PS32–(PDMAEMA121)2 and 0.12 wt% of PS30-b-PDMAEMA259 possess almost the same average droplets size (Fig. 4B and F). Thus, we can propose a reasonable hypothesis that the total emulsion droplet surface areas of the two o/w emulsions should be considered the same. Due to the larger area occupied by each Y-shaped polymer chain at the oil–water interface, a lesser amount of PS32–(PDMAEMA121)2 emulsifier was thus required than that of its linear counterpart.
Besides the o/w macroemulsion, PS–(PDMAEMA)2 emulsifiers could be applied for formation of w/o macroemulsion at a wider range of pH values. Our experiments revealed the formation of w/o emulsion strongly depended on the relative volume fraction of the oil phase or the water phase. For PS32–(PDMAEMA121)2, the formed macroemulsions were a w/o type once the volume fraction of toluene was more than 52% regardless of water pH and PS32–(PDMAEMA121)2 content (S3, ESI†). Decreasing the PDMAEMA block length led to reduction of the required volume fraction of toluene for w/o emulsion formation. For example, w/o macroemulsion was formed when the relative volume fraction of toluene was more than 50% when PS32–(PDMAEMA80)2 was used as a polymer emulsifier for macroemulsion (data not shown), and this might be ascribed to increased hydrophobicity of PS–(PDMAEMA)2 accompanying the decreasing of PDMAEMA block length.
Interfacial tension measurements were performed to gain additional insight into the activity of Y-shaped PS32–(PDMAEMA121)2 miktoarm star copolymer and linear PS30-b-PDMAEMA259 block copolymer at the oil–water interface. The toluene–water interfacial tension experiments were carried out by the Wilhelmy plate method. Interfacial tension experiments indicated that both kinds of emulsifiers demonstrated interfacial tension lowering behavior at the toluene–water interface. For example, the toluene–water (pH 5) interfacial tension was determined to be 28.1 mN m−1, which was consistent with previously reported 27.8 mN m−1 of toluene–water (pH 7).52,53 Interfacial tension decreased remarkably from 28.1 mN m−1 to 1.5 mN m−1 in the presence of 0.1 wt% of Y-shaped PS32–(PDMAEMA121)2 at pH 5, whereas the toluene–water (pH 5) interfacial tension was 3.7 mN m−1 in the presence of 0.1 wt% of linear PS30-b-PDMAEMA259.
3.3 Stimulus-responses of o/w emulsion stabilized by Y-shaped PS32–(PDMAEMA121)2 miktoarm star copolymer
It is well known that PDMAEMA exhibits both pH- and thermo-responsive properties.23 PDMAEMA aqueous solution shows reversible hydrophilic/hydrophobic transition with pH variation.23 The pKa of weak polyelectrolytic PDMAEMA is approximately 7 at room temperature.54 At lower pH, protonated PDMAEMA shows more hydrophilic characteristics, whereas deprotonation of tertiary amines gives the PDMAEMA arms more hydrophobic character and presents a higher affinity for oil when pH is above its pKa. At the same time, PDMAEMA possesses lower critical solution temperature (LCST) ranging from 32 to 60 °C, depending on its molecular weight or average polymerization degree.55 When the solution temperature is higher than its LCST, PDMAEMA polymer dissolved in neutral or weakly basic water becomes insoluble. These pH- and thermo-dependent hydrophilic/hydrophobic transition properties have conferred upon the PDMAEMA homopolymer and PDMAEMA-containing materials many important applications in wide fields, such as drug delivery and emulsions, etc.17,18,56–58 For example, Armes and his colleagues17 have reported a stimulus-responsive o/w Pickering emulsion stabilized by PS latexes. Due to the stimulus-response of poly-2-(dimethylamino)ethyl methacrylate-b-polymethyl methacrylate (PDMAEMA-b-PMMA) polymer chains absorbed on the surfaces of the polystyrene latexes, the generated emulsions showed temperature-dependent inversion and pH-induced demulsification.In the present work, the stimulus-responses of o/w emulsion stabilized by PS32–(PDMAEMA121)2 were investigated by altering pH or temperature. As expected, the o/w emulsion displayed rapid demulsification when the aqueous pH of the original o/w emulsion (Fig. 5A) was increased to about 9 by adding 1 mol L−1 NaOH aqueous solution, as shown in Fig. 5B. The demulsification resulted from increased hydrophobicity of PDMAEMA with the increase of pH. Complete phase separation was achieved after 24 h of standing at room temperature (Fig. 5C). After readjusting pH of the water to below pKa of PDMAEMA, such as 5, the separated oil could be completely re-emulsified by water (Fig. 5D). The average size of emulsion droplets of the regenerated emulsion was almost the same as that of the primary one (Fig. 5E and F). Upon heating the o/w emulsion (the volume ratio of toluene/water was 1) in a water bath at 55 °C, the o/w emulsion demonstrated two different phenomena. The primary emulsion shown in Fig. 6A was almost completely demulsified in a short time (∼5 min) if heating was applied alone or with gentle shaking by hand (Fig. 6B). At 55 °C, the PDMAEMA block becomes insoluble in pH 7 water and shows a higher affinity for oil. The stability of the o/w emulsion thus decreased, and coalesce of the original droplets resulted in the demulsification of the original emulsion. However, the original o/w emulsion inverted to an o/w/o multiple emulsion upon heating in the presence of moderate magnetic stirring (∼600 rpm) (Fig. 6C). An early report pointed out that the inversion of an emulsion was as the result of spontaneous curvature inversion of the emulsifier at an oil–water interface.23 Clearly, the spontaneous curvature inversion of the PS32–(PDMAEMA121)2 emulsifier absorbed at the oil–water interface could not be performed, and extra shearing action was necessarily required. Our control experiments showed that only a thinner layer of o/w emulsion was obtained by directly stirring an equal volume of pH 7 water and toluene containing PS32–(PDMAEMA121)2 at 55 °C either at a rate of 1000 rpm for 10 min or at a rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 1 min, as displayed in Fig. 6G and H. This suggested that the pre-adsorption of PS32–(PDMAEMA121)2 emulsifier at the oil–water interface was crucial to the thermo-induced inversion. Furthermore, we also noticed that this thermo-induced inversion could not be achieved for those emulsions prepared with acidic water (Fig. 6D–F); this might be a result of the difference in configuration of the PDMAEMA chain at the oil–water interface. The o/w/o multiple emulsion droplets showed a larger average size with a broader polydispersity (Fig. 6J) than those of the original o/w emulsion (Fig. 6I). In order to demonstrate the formed o/w/o multiple emulsion, CSLM was ulitized to image the emulsion droplets. For comparison, the droplets of the primary o/w emulsion were also imaged. The droplets of the primary o/w emulsion showed merged green and red channels (Fig. 6I, inset). The dispersed green droplets perfectly distinguished them from the red water bulk phase. The green and red color arisen from dissolved NBDAA in toluene and RhB dissolved in water, which were excited by a laser with wavelengths of 446 nm and 534 nm, respectively. After inversion, the red color droplets, which entrapped many small internal droplets with green color, were dispersed in the green bulk toluene, as shown in the inset of Fig. 6J, indicating the formation of o/w/o multiple macroemulsion.
000 rpm for 1 min, as displayed in Fig. 6G and H. This suggested that the pre-adsorption of PS32–(PDMAEMA121)2 emulsifier at the oil–water interface was crucial to the thermo-induced inversion. Furthermore, we also noticed that this thermo-induced inversion could not be achieved for those emulsions prepared with acidic water (Fig. 6D–F); this might be a result of the difference in configuration of the PDMAEMA chain at the oil–water interface. The o/w/o multiple emulsion droplets showed a larger average size with a broader polydispersity (Fig. 6J) than those of the original o/w emulsion (Fig. 6I). In order to demonstrate the formed o/w/o multiple emulsion, CSLM was ulitized to image the emulsion droplets. For comparison, the droplets of the primary o/w emulsion were also imaged. The droplets of the primary o/w emulsion showed merged green and red channels (Fig. 6I, inset). The dispersed green droplets perfectly distinguished them from the red water bulk phase. The green and red color arisen from dissolved NBDAA in toluene and RhB dissolved in water, which were excited by a laser with wavelengths of 446 nm and 534 nm, respectively. After inversion, the red color droplets, which entrapped many small internal droplets with green color, were dispersed in the green bulk toluene, as shown in the inset of Fig. 6J, indicating the formation of o/w/o multiple macroemulsion.
4. Conclusions
Y-shaped amphiphilic PS–(PDMAEMA)2 miktoarm star copolymer was successfully synthesized by combining RAFT polymerization with ATRP. When applied as a polymeric surfactant for w/o and o/w macroemulsion formation, the synthesized PS–(PDMAEMA)2 miktoarm star copolymers showed a higher emulsifying performance than that of their linear counterpart. The macroemulsion type was strongly dependent on the relative volume fraction of oil or water. Moreover, the required relative volume fraction of oil for w/o macroemulsion decreased with decreasing of the PDMAEMA block length. PS–(PDMAEMA)2 stabilized o/w macroemulsion showed pH-reduced demulsification and thermo-induced phase inversion. However, the curvature inversion of the PS–(PDMAEMA)2 emulsifier at the oil–water interface could not be spontaneously accomplished, and moderate stirring was necessary required. Furthermore, a thermo-induced phase inversion could only be realized for emulsions with pH 7 water, and a more acidic water was not favorable. This type of manipulated emulsion is expected to provide useful guidance in the fields of oil recovery, catalysis, and perhaps other applications.Acknowledgements
This work was financially supported by the National Natural Scientific Foundation of China (21174118, 21404036) and Open Project of Hunan Provincial University Innovation Platform (15K123).References
- T. Tadros and B. Vincent, Emulsion stability, in Encyclopedia of Emulsion Technology, Marcel Dekker, Inc., New York, 1983 Search PubMed.
- S. U. Pickering, J. Chem. Soc., Trans., 1907, 91, 2001–2021 RSC.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108, 303–318 CrossRef PubMed.
- T. F. Tadros, Emulsion formation and stability, John Wiley & Sons, 2013 Search PubMed.
- B. K. Paul and S. P. Moulik, Curr. Sci., 2001, 80, 990–1001 CAS.
- J. K. Oh, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6983–7001 CrossRef CAS.
- P. Ghosh and R. Murthy, Curr. Drug Delivery, 2006, 3, 167–180 CrossRef CAS.
- K. Jadhav, I. Shaikh, K. Ambade and V. Kadam, Curr. Drug Delivery, 2006, 3, 267–273 CrossRef CAS.
- D. K. Sarker, Curr. Drug Delivery, 2005, 2, 297–310 CrossRef CAS.
- M. Porras, C. Solans, C. Gonzalez and J. Gutierrez, Colloids Surf., A, 2008, 324, 181–188 CrossRef CAS.
- K. Landfester, A. Musyanovych and V. Mailänder, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 493–515 CrossRef CAS.
- P. A. Psathas, S. R. da Rocha, C. T. Lee, K. P. Johnston, K. Lim and S. Webber, Ind. Eng. Chem. Res., 2000, 39, 2655–2664 CrossRef CAS.
- S. Davis, J. Hadgraft, K. Palin and P. Becher, Encyclopedia of emulsion technology, Marcel Dekker, New York, 1985, vol. 2, p. 159 Search PubMed.
- B. P. Binks, J. Philip and J. A. Rodrigues, Langmuir, 2005, 21, 3296–3302 CrossRef CAS PubMed.
- Y. Chevalier and M.-A. Bolzinger, Colloids Surf., A, 2013, 439, 23–34 CrossRef CAS.
- I. Akartuna, A. R. Studart, E. Tervoort, U. T. Gonzenbach and L. J. Gauckler, Langmuir, 2008, 24, 7161–7168 CrossRef CAS PubMed.
- B. P. Binks, R. Murakami, S. P. Armes and S. Fujii, Angew. Chem., 2005, 117, 4873–4876 CrossRef.
- E. Read, S. Fujii, J. Amalvy, D. Randall and S. Armes, Langmuir, 2004, 20, 7422–7429 CrossRef CAS PubMed.
- G. Riess, Prog. Polym. Sci., 2003, 28, 1107–1170 CrossRef CAS.
- K. P. Johnston, D. Cho, S. R. DaRocha, P. A. Psathas, W. Ryoo, S. E. Webber, J. Eastoe, A. Dupont and D. C. Steytler, Langmuir, 2001, 17, 7191–7193 CrossRef CAS.
- P. Perrin, Langmuir, 1998, 14, 5977–5979 CrossRef CAS.
- Y. S. Ko, M. V. Circu, T. Geiger, S. Dünki, F. A. Nüesch and D. M. Opris, RSC Adv., 2014, 4, 35027–35034 RSC.
- F. Marchal, A. Roudot, N. Pantoustier, P. Perrin, J. Daillant and P. Guenoun, J. Phys. Chem. B, 2007, 111, 13151–13155 CrossRef CAS PubMed.
- Y.-J. Hwang, C. Oh and S.-G. Oh, J. Controlled Release, 2005, 106, 339–349 CrossRef CAS PubMed.
- R. Jerome and P. Teyssie, J. Appl. Polym. Sci., 1981, 26, 343–351 CrossRef.
- N. Hadjichristidis, M. Pitsikalis, S. Pispas and H. Iatrou, Chem. Rev., 2001, 101, 3747–3792 CrossRef CAS PubMed.
- K. Khanna, S. Varshney and A. Kakkar, Polym. Chem., 2010, 1, 1171–1185 RSC.
- W. Zhang, W. Zhang, J. Zhu, Z. Zhang and X. Zhu, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 6908–6918 CrossRef CAS.
- Y. Yamazaki, N. Ajioka, A. Yokoyama and T. Yokozawa, Macromolecules, 2009, 42, 606–611 CrossRef CAS.
- Z. Li, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2004, 37, 8933–8940 CrossRef CAS.
- R. Matmour and Y. Gnanou, J. Am. Chem. Soc., 2008, 130, 1350–1361 CrossRef CAS PubMed.
- W. Li, Y. Yu, M. Lamson, M. S. Silverstein, R. D. Tilton and K. Matyjaszewski, Macromolecules, 2012, 45, 9419–9426 CrossRef CAS.
- Q. Chen, X. Cao, Y. Xu and Z. An, Macromol. Rapid Commun., 2013, 34, 1507–1517 CrossRef CAS PubMed.
- C. R. Becer, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 4900–4908 CrossRef CAS PubMed.
- J. Yang, D. Zhang, S. Jiang, J. Yang and J. Nie, J. Colloid Interface Sci., 2010, 352, 405–414 CrossRef CAS PubMed.
- Y. Cai, C. Burguiere and S. P. Armes, Chem. Commun., 2004, 802–803 RSC.
- G. Maglio, F. Nicodemi, C. Conte, R. Palumbo, P. Tirino, E. Panza, A. Ianaro, F. Ungaro and F. Quaglia, Biomacromolecules, 2011, 12, 4221–4229 CrossRef CAS PubMed.
- K. Yoon, H. C. Kang, L. Li, H. Cho, M.-K. Park, E. Lee, Y. H. Bae and K. M. Huh, Polym. Chem., 2015, 6, 531–542 RSC.
- H. Yin, S.-W. Kang and Y. H. Bae, Macromolecules, 2009, 42, 7456–7464 CrossRef CAS.
- J. Kulis, Z. Jia and M. J. Monteiro, Macromolecules, 2012, 45, 5956–5966 CrossRef CAS.
- H. Liu, J. Xu, J. Jiang, J. Yin, R. Narain, Y. Cai and S. Liu, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1446–1462 CrossRef CAS.
- B. Zhang, Y. Li, W. Wang, L. Chen, S. Wang and J. Wang, Polym. Bull., 2009, 62, 643–655 CrossRef CAS.
- A. Vora, K. Singh and D. C. Webster, Polymer, 2009, 50, 2768–2774 CrossRef CAS.
- J. Rao, Y. Zhang, J. Zhang and S. Liu, Biomacromolecules, 2008, 9, 2586–2593 CrossRef CAS PubMed.
- L.-Y. Li, W.-D. He, J. Li, B.-Y. Zhang, T.-T. Pan, X.-L. Sun and Z.-L. Ding, Biomacromolecules, 2010, 11, 1882–1890 CrossRef CAS PubMed.
- S. Petrova, C. G. Venturini, A. Jäger, E. Jäger, M. Hrubý, E. Pavlova and P. Štěpánek, RSC Adv., 2015, 5, 62844–62854 RSC.
- J. Morsbach, A. Natalello, J. Elbert, S. Winzen, A. Kroeger, H. Frey and M. Gallei, Organometallics, 2013, 32, 6033–6039 CrossRef CAS.
- J. Liu, Z. Nie, Y. Gao, A. Adronov and H. Li, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7187–7199 CrossRef CAS.
- Z. Zhang, G. Liu and S. Bell, Macromolecules, 2000, 33, 7877–7883 CrossRef CAS.
- Y. Cai and S. P. Armes, Macromolecules, 2005, 38, 271–279 CrossRef CAS.
- Y. Cai, Y. Tang and S. P. Armes, Macromolecules, 2004, 37, 9728–9737 CrossRef CAS.
- R. Prokop, M. Hair and A. Neumann, Macromolecules, 1996, 29, 5902–5906 CrossRef CAS.
- M. K. Sanyal, V. V. Agrawal, M. K. Bera, K. Kalyanikutty, J. Daillant, C. Blot, S. Kubowicz, O. Konovalov and C. Rao, J. Phys. Chem. C, 2008, 112, 1739–1743 CAS.
- V. Bütün, S. Armes and N. Billingham, Polymer, 2001, 42, 5993–6008 CrossRef.
- S. Liu, J. V. Weaver, Y. Tang, N. C. Billingham, S. P. Armes and K. Tribe, Macromolecules, 2002, 35, 6121–6131 CrossRef CAS.
- S. Agarwal, Y. Zhang, S. Maji and A. Greiner, Mater. Today, 2012, 15, 388–393 CrossRef CAS.
- A. Car, P. Baumann, J. T. Duskey, M. Chami, N. Bruns and W. Meier, Biomacromolecules, 2014, 15, 3235–3245 CrossRef CAS PubMed.
- J. Hu, G. Zhang, Z. Ge and S. Liu, Prog. Polym. Sci., 2014, 39, 1096–1143 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16399d |
| This journal is © The Royal Society of Chemistry 2015 |