A supramolecular assembling of zinc porphyrin with a π-conjugated oligo(phenylenevinylene) (oPPV) molecular wire for dye sensitized solar cell†
Asterios Charisiadisa,
Christina Stangela,
Vasilis Nikolaoua,
Mahesh S. Royb,
Ganesh D. Sharmac and
Athanassios G. Coutsolelos*a
aDepartment of Chemistry, University of Crete, Laboratory of Bioinorganic Chemistry, Voutes Campus, P. O. Box 2208, 71003 Heraklion, Crete, Greece. E-mail: acoutsol@uoc.gr
bDefence Laboratory, Jodhpur, Raj. 342011, India
cR & D Centre for Engineering and Science, JEC Group of Colleges, Jaipur Engineering College Campus, Kukas, Jaipur, Raj. 303101, India. E-mail: gdsharma273@gmail.com
First published on 12th October 2015
Abstract
A novel π-conjugated oligo(phenylenevinylene) (oPPV) (or LC) was prepared, as a new organic dye for dye-sensitized solar cells (DSSC), which contains a cyanoacrylic acid group on one end and a pyridyl group on the other. Solar cells sensitized by LC were fabricated, and were found to exhibit a power conversion efficiency (PCE) value of 2.45%. Furthermore, we describe the formation of a supramolecular dyad (ZnTPP–LC) via a metal–ligand bond between LC, since its pyridyl group allows it to interact with several metal centers, and zinc-tetraphenyl-porphyrin (ZnTPP) onto the photoelectrode's TiO2 surface of the solar cell. More specifically, LC was bound at first onto TiO2 with its cyanoacrylic acid anchoring group, and then a metal–ligand supramolecular bond was formed, with the addition of a porphyrinic solution, between the nitrogen atom of LC's pyridyl group and the zinc. ZnTPP–LC solar cell was then fabricated resulting in a record PCE value of 5.27% concerning the supramolecular DSSCs. As shown by photovoltaic measurements (J–V curves) and incident photon to current conversion efficiency (IPCE) spectra of the two solar cells, the higher PCE value of the supramolecular one can be attributed to its enhanced photovoltaic parameters, and particularly its enhanced short circuit current (Jsc). This Jsc improvement is due to ZnTPP–LC's higher light-harvesting efficiency and the larger electron injection of both ZnTPP and LC into TiO2's conduction band (CB) of the corresponding solar cell. These results are in accordance with electrochemical impedance spectra (EIS) of the DSSCs, which revealed longer electron lifetime, higher charge recombination resistance and shorter electron transport time for the solar cell based on ZnTPP–LC as compared to the one sensitized by LC.
Introduction
In the last few decades, solar energy has been demonstrated to be a promising alternative to fossil fuels, and dye-sensitized solar cells (DSSCs) have attracted considerable interest owing to their relatively high power-conversion efficiencies, low production costs, ease of fabrication and modifiable aesthetic features such as vivid colours and high transparency.1–8 Nowadays, DSSCs fabricated from environmentally friendly and inexpensive materials appear to be a very promising technology for low-cost and highly efficient solar energy conversion.9,10 A typical device of this type consists of a wide-band-gap semiconductor photoanode (usually TiO2) sensitized by a molecular dye, a redox electrolyte, and a platinum counter electrode. The sensitizer plays a key role in the operation of these devices because it is responsible for the absorption of solar radiation, the generation of excited electrons, and their injection into the TiO2 conduction band (CB). Ru complex photosensitizers such as N3, N719 and black dye exhibit efficiencies higher than 10%, and this is the reason why they have been so widely used.11,12 The performance of DSSCs based on organic dyes have also recently been remarkably improved, as for example the highly efficient performance from a π-conjugated oligo-phenylenevinylene organic dye, which contains a diphenylamine donor group and a cyanoacrylic acid anchoring group.13 Furthermore, considerable efforts have been devoted to the construction of efficient sensitizers with typical donor–π–acceptor (D–π–A) “push-pull” structures.14,15 In this respect, porphyrins have demonstrated their potential owing to the strong absorption in a large wavelength range and facile structural modification,16–24 as for example it was reported a PCE value of 13% from a benzothiadiazole functionalized porphyrin.25,26 To the best of our knowledge, this is the first example in the literature where a complex is formed between a chromophore and a molecular wire bearing an anchoring group. The nature of the coordination bond that is formed between the zinc and the pyridyl group of the molecular wire provides a new supramolecular type architecture, with very promising properties.Despite all the previous aspects, an intrinsic drawback for typical porphyrin-based dyes still remains their lack of absorption in the IR region, as well as in the region between the Soret and the Q bands, which hampers further improvement of the DSSC efficiencies,25,27–29 and thus examples of porphyrin dyes exhibiting DSSC efficiencies higher than 10% are still very rare.25,27–30 In an effort to further improve the performance of porphyrin based solar cells, multi-porphyrin arrays consisting of covalently linked porphyrin macrocycles or other units, such as Bodipy, linked either through suitable π-conjugated groups or directly, have been investigated as sensitizers.31–34 There are several reports of covalently linked electron donor–bridge–acceptor (D–B–A) conjugates for numerous applications.35–38 However, the synthesis of extended chromophores and covalently linked electron D–B–A systems is challenging and therefore these materials are far from ideal for practical applications.
As it is known, in nature, self-assembly through non-covalent binding motifs,39 such as hydrogen bonding and metal–ligand coordination, plays a dominant role. For example, photosynthetic antenna reaction center pigments use such intermolecular forces to precisely arrange the donor–acceptor entities in a protein matrix, exhibiting a cascade of vectorial energy and electron transfer processes.40 Inspired by this revelation, several groups have constructed supramolecular photosynthetic architectures to mimic the photoinduced energy and electron transfer processes, with an ultimate goal of building efficient light-energy-harvesting devices based on these biomimetic principles.41–49 Supramolecular solar cells based on biomimetic principles could serve as an alternative to semiconductor-based ones for renewable energy production, though their efficiencies remain relatively low. The binding motif that has been widely used for the fabrication of supramolecular DSSCs is the versatile metal–ligand bond.50–55 On this aspect, D'Souza and coworkers used four ligands with different pKa values, which bear from one side a carboxylic group, that enables the functionalization of the TiO2 surface, and from the other a pyridyl or imidazolyl group that enables it to interact with a metal center via a supramolecular metal–ligand bond.56 Thus, it becomes possible to connect an electron donor with an acceptor resulting in a D–π–A type supramolecular system.
Herein we demonstrate the synthesis of a novel π-conjugated oligo(phenylenevinylene) (oPPV) moiety (LC), which could be used as a promising organic dye in dye-sensitized solar cells (DSSCs). The DSSC based on LC organic dye showed a PCE value of 2.45%. LC contains a cyanoacrylic acid as anchoring group, a pyridyl group that allows the axial interaction with a bunch of metallated electron donor dyes and six dodecyloxy groups on its periphery preventing aggregations. In this work, we describe the fabrication of a supramolecular DSSC with LC, which acts as a π-bridge, and zinc-tetraphenylporphyrin (ZnTPP) (Scheme 1), that plays the role of an electron donor, as they form a novel supramolecular dyad onto the TiO2 surface. More specifically, LC was firstly bound on TiO2 and then the electrode was immersed in a ZnTPP solution in order for zinc to form a metal–ligand bond with the oPPV's pyridyl group (Scheme 2), leading to the supramolecular dyad ZnTPP–LC, structurally similar to the record holder one of the organic dyes,13 and then its performance was monitored. The choice of the tetraphenylporphyrin is due to its high chemical activity and capacity to form stable coordination bonded derivatives with pyridyl moieties. Tetraphenylporphyrin metallated with zinc is an excellent donor unit with relatively straightforward synthesis. The main advantage of this approach is the highly effective prevention of aggregations, due to the presence of six dodecyloxy groups and the directionality of the metal–ligand bond. We observed an important improvement on the efficiency of the cell, since a PCE value equal to 5.27% was achieved.
Experimental detail
Materials and techniques
Solvents and reagents were purchased from the usual commercial sources and used as received, unless otherwise stated. Also, 4-{(E)-4-[(E)-2,5-bis(dodecyloxy)-4-((E)-2-(pyridin-4-yl)vinyl)styryl]-2,5-bis(dodecyloxy)styryl}-2,5-bis(dodecyloxy)benzaldehyde (1) was synthesized from our group.50NMR spectra
NMR spectra were recorded on a Bruker AVANCE III-500 MHz spectrometer using solutions in deuterated solvents by using the solvent peak as the internal standard.Mass spectra
High-resolution mass spectra (HRMS) were recorded on a Bruker UltrafleXtreme MALDI-TOF/TOF spectrometer.Photophysical measurements
All UV-vis absorption spectra were measured on a Shimadzu UV-1700 spectrophotometer using 10 mm path-length cuvettes.Electrochemistry
Both cyclic voltammetry (CV) and square-wave voltammetry (SW) measurements were performed at room temperature by using an AutoLab PGSTAT20 potentiostat. Freshly distilled and deoxygenated THF was used while carrying out CV and SW measurements, with scan rate 100 mV s−1, with a solute concentration of 1.0 mM in the presence of tetrabutylammonium tetrafluoroborate (0.1 M) as supporting electrolyte. A three-electrode cell setup was used with a saturated calomel (SCE) reference electrode, a platinum working electrode and a platinum wire counter electrode.Computational details
Density functional theory (DFT) calculations57 were performed using the GAUSSIAN 03 program suite.58 Gas phase geometry optimizations were carried with B3LYP59,60 functionals using the LANL2DZ basis set for Zn atoms and the 6-31G(d) basis sets for lighter atoms. The optimized minimum-energy structures were verified by vibrational frequency analysis calculation. Tomasi's Polarizable Continuum Model (PCM)61 was applied for describing the solvent effect (dichloromethane) with standard dielectric constant e = 8.93. The input geometries and molecular orbitals were modeled using ChemCraft software (version 1.6).DSSC fabrication and characterization
The working and counter electrodes consisting of TiO2 and thermally platinum films, respectively, were deposited onto F-doped tin oxide (FTO) conducting glass substrates by the same technique as reported earlier.62 For the fabrication of the supramolecular DSSC, the prepared TiO2 substrate (particle size of TiO2 is 25 nm, thickness of TiO2 film is 12 μm) was first immersed in a THF solution of the LC dye (0.20 mM) for 4 h, which allowed the dye to anchor onto the TiO2 surface and then it was washed with ethanol for the removal of the unbound dye. The dye-functionalized surface was then immersed in a CH2Cl2 solution of ZnTPP (2 mM) in order for the ZnTPP–LC dyads to be formed on the TiO2 surface. Thus, a coordination bond between the pyridyl and zinc forms the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between the molecular wire and the zinc porphyrin during the second step, as observed from the dye loading amounts. The sensitized working electrode was assembled with the Pt coated FTO electrode into a sandwich type cell and sealed with the hot-melt polymer Surlyn. To complete the DSSC fabrication, the electrolyte solution containing LiI (0.05 M), I2 (0.03 M), 1 methyl-3-n-propylimidazolium iodide (0.6 M) and tert-butylpyridine (0.5 M in a mixture of acetonitrile and valeronitrile, 85
1 complex between the molecular wire and the zinc porphyrin during the second step, as observed from the dye loading amounts. The sensitized working electrode was assembled with the Pt coated FTO electrode into a sandwich type cell and sealed with the hot-melt polymer Surlyn. To complete the DSSC fabrication, the electrolyte solution containing LiI (0.05 M), I2 (0.03 M), 1 methyl-3-n-propylimidazolium iodide (0.6 M) and tert-butylpyridine (0.5 M in a mixture of acetonitrile and valeronitrile, 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 volume ratio) was introduced into the space between the two electrodes through a drilled hole in the platinum coated FTO by vacuum backfilling. The DSSC sensitized by the LC organic dye was also fabricated, under the same conditions. We also measured the dye loading on both DSSCs sensitized by LC and LC-DSSC, and it was found to be 5.31 × 10−8 mol cm−2 for the LC sensitized cell and 5.40 × 10−8 mol cm−2 for the supramolecular DSSC. It is very clear that the two dye loading values are slightly different, since ZnTPP does not contain any anchoring unit and it only coordinated on the pyridyl end of the wire (LC) and accelerates the electron injection. The active area of both fabricated DSSCs was found to be around 0.25 cm2.
15 volume ratio) was introduced into the space between the two electrodes through a drilled hole in the platinum coated FTO by vacuum backfilling. The DSSC sensitized by the LC organic dye was also fabricated, under the same conditions. We also measured the dye loading on both DSSCs sensitized by LC and LC-DSSC, and it was found to be 5.31 × 10−8 mol cm−2 for the LC sensitized cell and 5.40 × 10−8 mol cm−2 for the supramolecular DSSC. It is very clear that the two dye loading values are slightly different, since ZnTPP does not contain any anchoring unit and it only coordinated on the pyridyl end of the wire (LC) and accelerates the electron injection. The active area of both fabricated DSSCs was found to be around 0.25 cm2.
The current–voltage (J–V) characteristics of the DSSCs under illumination were measured using a Keithley source meter and a solar simulator coupled with a 150 W xenon lamp and an AM optical filter to give an illumination intensity of 100 mW cm−2 on the surface of the cells. The electrochemical impedance spectra (EIS) in the dark were recorded by using an electrochemical workstation (Auto lab PGSTAT) with a frequency response analyzer. The frequency range was from 10 mHz to 100 KHz, and an ac potential of 10 mV was used. A dc bias equivalent to the open-circuit voltage of the DSSC was applied. The impedance data were analyzed by the Z-View software with an appropriate equivalent circuit. The incident photon to current conversion efficiency (IPCE) was measured as a function of wavelength with a xenon lamp, a monochromator, and a Keithley source meter at 100 mW cm−2. The photocurrent was measured under short circuit conditions. A standard silicon photodiode performed the intensity calibration for the IPCE measurement.
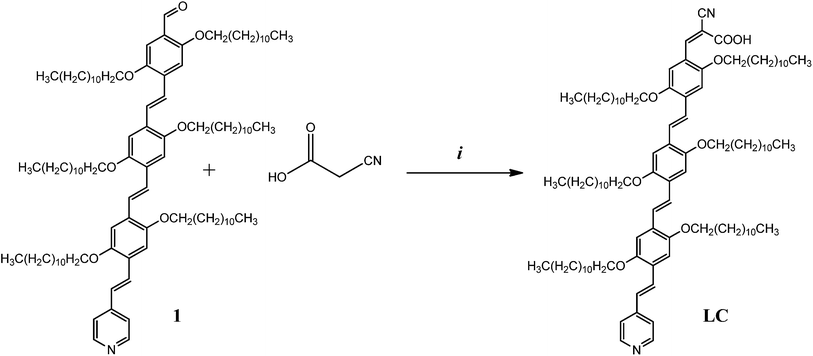
Synthesis of (Z)-3-(4-((E)-2,5-bis(dodecyloxy)-4-((E)-2-(dodecyloxy)-4-((E)-2-(pyridin-4-yl)vinyl)-5-((undecyloxy)methyl)styryl)styryl)-2,5-bis(dodecyloxy)phenyl)-2-cyanoacrylic acid (LC)
An acetic acid (CH3COOH) (5 mL) and tetrahydrofuran (THF) (2 mL) mixed solution of (1) (25 mg, 0.0165 mmol) and 2-cyanoacetic acid (11.21 mg, 0.1317 mmol) was stirred at 115 °C for 24 h under nitrogen and in the presence of piperidine (4 μL). The resulting solution was evaporated to dryness under vacuum. The crude residue was purified by column chromatography over silica gel using CH2Cl2/MeOH (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 v/v) as an eluent to obtain the (LC) as a red solid (yield 20 mg, 76.47%).
6 v/v) as an eluent to obtain the (LC) as a red solid (yield 20 mg, 76.47%).
Results and discussion
Synthesis and characterization
The synthetic route, which was followed for the preparation of oPPV LC, is shown in Scheme 3. More specifically, LC was synthesized according to the Knoevenagel condensation methodology,63 via the reaction of 4-((E)-4-((E)-2,5-bis(dodecyloxy)-4-((E)-2-(pyridin-4-yl)vinyl)styryl)-2,5-bis(dodecyloxy) styryl)-2,5-bis(dodecyloxy)benzaldehyde (1)57 and 2-cyanoacetic acid in a mixture of acetic acid (CH3COOH)/tetrahydrofuran (THF) under refluxing conditions and in the presence of a catalytic amount of piperidine, followed by chromatographic purification. Compound LC was designed as an axially symmetrical ligand, capable of providing direct electronic communication between the cyanoacrylic (–CNCOOH) anchoring group and the pyridyl N-atom. Furthermore, upon complexation with ZnTPP, the electronic communication could expand between –CNCOOH group and the metal center of ZnTPP. ZnTPP was prepared by following a previously reported procedure.64 The dyad ZnTPP–LC was formed with non-covalent interactions throughout the fabrication of the DSSC devices.The purity of dye LC was confirmed by 1H and 13C NMR (Fig. S1a and b and S2a and b†), UV-vis absorption, matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrum (Fig. S3†) and elemental analysis. Its 1H NMR spectrum confirms the E configuration of the double bonds by a coupling constant of exactly 16.5 Hz for the AB system that corresponds to the vinylic protons of the oPPV moiety. Moreover, MALDI-TOF mass spectrum showed the expected molecular ion peak: m/z 1585.65 (LC). Due to the presence of the dodecyloxy groups, both the LC oPPV and ZnTPP–LC dyad are very soluble in a variety of organic solvents with different polarities such as CH2Cl2, CHCl3, THF and toluene.
The terminal cyanoacrylic group enables oPPV's coordination to a variety of Lewis acids sites; hence, the LC compound has the prospective to anchor onto the TiO2 surface of the DSSC photoelectrode. In addition, the presence of six long alcoxy (–OCH2(CH2)10CH3) chains at the periphery of both the LC oPPV and the ZnTPP–LC dyad prevents the formation of porphyrin aggregates on the TiO2 surface, which is a major reason for the low performance of porphyrin-based DSSCs.
Photophysical properties
The optical absorption spectrum of the ZnTPP–LC dyad in CH2Cl2 solution is shown in Fig. 1a. The optical absorption spectra of the constituent chromophores (ZnTPP and LC) are also shown in Fig. 1a, for comparison reasons. The absorption spectrum of ZnTPP–LC, which shows the characteristic peaks of both ZnTPP and LC moieties, indicates negligible electronic interactions between those two units in the ground state of the supramolecular dyad. Upon coordination of LC on ZnTPP, we observed a slight increase in all the molar extinction coefficients concerning the four peaks shown in ZnTPP–LC dyad's spectrum. All the optical data that resulted from the three UV-vis spectra, which were described above, are listed in Table 1. | ||
| Fig. 1 Optical absorption spectra of (a) ZnTPP, LC and ZnTPP–LC in solution and (b) LC and ZnTPP–LC adsorbed onto TiO2 surface. | ||
The absorption spectra of LC and ZnTPP–LC dyad adsorbed onto the TiO2 film (Fig. 1b). Those two spectra are similar to the absorption spectra of LC and ZnTPP–LC in solution, but exhibit a small red shift and broadening, reflecting slight aggregation.
Electrochemical studies
In order for a complex to be considered an effective sensitizer in a DSSC, two processes should be considered: efficient dye regeneration and electron injection. Those two processes require suitably aligned lowest unoccupied molecular orbital (LUMO) and highest occupied molecular orbital (HOMO) energy levels. Firstly, concerning the first process, the HOMO energy level of the dye should be lower than the corresponding redox potential of the electrolyte redox couple, while, concerning the second process, its LUMO energy level should be higher than the TiO2 CB edge.7,65The HOMO and LUMO energy levels of LC were determined by both square-wave voltammetry measurements (Fig. S4†) and cyclic voltammetry measurements (Fig. S4†). Also, the HOMO and LUMO energy levels of the ZnTPP are known from the bibliography.66 So, the peak potentials for both those compounds are summarized in Table 2. The HOMO energy levels were found to be 1.09 and 0.82 V vs. NHE, respectively, and they are lower than the oxidation potential for the I3−/I− redox couple. Additionally, their LUMO energy levels were found to be −1.49 and −1.31 V vs. NHE, respectively, which are located higher than the TiO2 CB (−0.5 V vs. NHE). Therefore, electron injection from the excited states of both dyes into the TiO2 CB edge and regeneration of the photooxidized dyes by transferring electrons from I3− of the electrolyte, are favored. Finally, it was not able for us to determine the HOMO and LUMO energy levels of the ZnTPP–LC dyad, via neither cyclic nor square-wave voltammetry, due to the weakness of this metal–ligand bond. Nevertheless, since the HOMO and LUMO energy levels of the constituent chromophores were found to fulfill the DSSC requirements, we can assume that their dyad should also be a good DSSC dye-candidate.
DFT calculations
Theoretical calculations within the framework of density functional theory were employed in order to further investigate the electronic properties of the final compound ZnTPP–LC. The total number of atoms of ZnTPP–LC is too large for DFT calculations in our computer cluster, therefore we simplified the structure by replacing the long dodecyloxy groups of the phenyl substituents of the LC with methoxy groups, which should not influence their HOMO and LUMO energy levels.36 The gas-phase optimized coordinates are given in the ESI in Table S1† and the gas-phase optimized structure is depicted in Fig. 2. In addition, a different view perspective of the gas-phase geometry structure of ZnTPP–LC is presented in ESI (Fig. S5†). We observe that the phenyl groups of the porphyrin ring are almost perpendicular to the phenyl substituents of the LC. Moreover the phenyl groups of the LC adopt a co-planar orientation to each other. For the optimized structure of ZnTPP–LC the corresponding HOMO and LUMO energies, the HOMO–LUMO gap and the dipole moment are listed in Table 3. The HOMO–LUMO gap was calculated in dichloromethane solution and was found to be 2.12 eV. The electronic density distribution and the corresponding energies of the frontier molecular orbitals (FMOs) are depicted in Fig. 3. In two of the highest molecular orbitals (HOMO and HOMO−2) the electron density is spread only on the central of the porphyrin unit. In case of the HOMO−1 though, the electron density is mainly located on the second and third phenyl substituents of the LC, with some additional distribution on the first and fourth phenyl group. Regarding the lowest molecular orbitals we observe that LUMO and LUMO+1 are entirely localized on the bridging phenyl groups and on the cyanoacrylic acid group, unlike the LUMO+2 that is spread over the porphyrin unit. The majority of the above mentioned distributions, namely HOMO, HOMO−2, LUMO and LUMO+1 indicate that intramolecular electron transfer is favorable. Hence, ZnTPP–LC could be described as a “push-pull” D–π–A (D: donor, A: acceptor) compound that is a promising candidate for DSSCs applications. The phenyl substituents of the LC constitute the π-conjugated system that assist the electron transfer from the zinc porphyrin (donor) to the terminating cyanoacrylic group (acceptor). The cyanoacrylic is the anchoring group of the ZnTPP–LC that promotes the electron injection into the conduction band of the TiO2. Finally, a collection of the corresponding HOMO and LUMO energies as well as the HOMO–LUMO gap and the dipole moment are listed in Table 3. | ||
| Fig. 2 Gas phase geometry optimized structure of ZnTPP–LC. Carbon, nitrogen, hydrogen, oxygen and zinc atoms correspond to grey, blue, white, red and green spheres, respectively. | ||
| Compound | HOMO (eV) | LUMO (eV) | HL (eV) | μ (D) |
|---|---|---|---|---|
| ZnTPP–LC | −4.807 | −2.689 | 2.12 | 5.48 |
 | ||
| Fig. 3 Frontier molecular orbitals of ZnTPP–LC and corresponding energy levels from DFT calculation in CH2Cl2. | ||
Photovoltaic properties
The current–voltage characteristics of the DSSC based on ZnTPP–LC are shown in Fig. 4a. The J–V characteristics of the DSSC based on LC are also shown in Fig. 4a. The photovoltaic parameters of these DSSCs are summarized in Table 4. The DSSC sensitized by the ZnTPP–LC dyad showed a much higher Jsc value (11.54 mA cm−2) and a higher Voc value (0.66 V) than those displayed by the LC based DSSC. Consequently, the DSSC based on the ZnTPP–LC dyad showed a power conversion efficiency (PCE) of 5.27%, which is higher than the PCE value of the LC, based DSSC (2.45% with Jsc = 7.55 mA cm−2, Voc = 0.56 V and FF = 0.58). The higher PCE of the cell based on our dyad can be attributed to its better light harvesting efficiency (LHE) over the wider wavelength region. The higher Jsc may also be related to the fact that upon photosensitization the dyad can undergo two step vectorial electron transfer to produce long-lived charge separated states in which electrons (in TiO2) and holes (in ZnTPP) are further separated, whereas the charge recombination could be much faster in the cell sensitized by our novel organic dye due to the closer proximity of electrons and holes in the charge separated states. | ||
| Fig. 4 (a) Current–voltage characteristics under illumination, and (b) IPCE spectra of the DSSCs based on LC, and ZnTPP–LC. | ||
| Compound | Jsc (mA cm−2) | Voc (V) | FF | PCE |
|---|---|---|---|---|
| LC | 7.55 | 0.56 | 0.58 | 2.45 |
| ZnTPP–LC | 11.58 | 0.66 | 0.69 | 5.27 |
The higher Jsc of the DSSC sensitized by ZnTPP–LC dye is one of the major reasons for its higher PCE value. Since the Jsc of the DSSC depends strongly on the incident photon to current efficiency (IPCE) response, the difference in the values of Jsc of these DSSCs can be reflected in the IPCE spectra (Fig. 4b). The IPCE spectra of the DSSC sensitized by the ZnTPP–LC dyad are broader as compared to DSSC based on the LC dye. The photocurrent generated in the 400–430 nm region is caused by the excitation of ZnTPP (Soret band), whereas, excitation of LC triggers the current generation in the 430–530 nm region, where LC exhibits strong absorption (Fig. 1a). In addition, excitation of both ZnTPP and LC contributes to the photocurrent in 530–650 nm region (Q bands of ZnTPP). The IPCE value of the DSSC sensitized by ZnTPP–LC dye reaches a peak at 35 and 40% at 422 nm and 486 nm, respectively, i.e. Soret band of ZnTPP and absorption peak of LC. In contrast, the DSSC sensitized by dye produces photocurrent only at wavelengths where LC absorbs light and its IPCE is lower compared to that of the ZnTPP–LC based cell. The IPCE spectra of the DSSCs closely resemble the absorption spectra of the LC or ZnTPP–LC sensitized, in each case, TiO2 photoelectrode. It can be seen in Fig. 4b, that the excitation of the cell at the wavelengths where the lower energy Q-bands absorb, also produces the current, indicating that it is also possible for the photoinduced charge transfer to be initiated that way as well. Furthermore, the DSSC based on the ZnTPP–LC complex showed broader IPCE spectrum, which also indicates the formation of ZnTPP aggregations was successfully prevented due to the presence of the LC moieties, where each one bears six dodecyloxy chains on their periphery. The values of Jsc estimated from the integration of the IPCE spectra were found to be 7.43 mA cm−2 and 11.44 mA cm−2, for the LC and ZnTPP–LC based solar cells respectively, which are consistent with the values estimated form J–V characteristics (Table 4). The increase in the Jsc value in the case of the ZnTPP–LC complex can also be attributed to its higher dye loading onto the TiO2 surface.
Another parameter that plays an important role for the higher PCE value of the supramolecular DSSC compared to the organic solar cell is the open circuit voltage (Voc), which shows an improved value for the first cell. In general, the Voc of a DSSC depends on the difference between the quasi Fermi level of TiO2 and the redox potential of the electrolyte (Eredox) and is expressed by the followed equation:
Additionally, the dark current gives information about the recombination of the injected electrons with the I3− form of the electrolyte in a DSSC. The J–V characteristics under darkness of both DSSCs are shown in Fig. 5 and reveal that dark current is lower for the DSSC sensitized by ZnTPP–LC as compared to the other one, indicating the suppression of the charge recombination rate.
 | ||
| Fig. 5 Current–voltage (J–V) characteristics under dark conditions for DSSCs sensitized with LC and ZnTPP–LC. | ||
The electrochemical impedance spectroscopy (EIS) has been a very powerful tool in understanding of all interfacial processes in DSSCs.67,68 To understand the relationship between the photovoltaic response and the charge transfer processes in the DSSCs, we have recorded the EI spectra of the DSSCs in dark conditions, applying an external dc biasing equivalent to open circuit voltage of the DSSC. The typical Nyquist plots of EIS of the two DSSCs are shown in Fig. 6a. In general, the Nyquist plots of the EI spectra comprise three arcs that are associated with the charge transfer at the counter electrode/electrolyte interface (high frequency region), the electron transfer kinetics at the TiO2/dye/electrolyte interface (middle frequency region), and the Nernstian diffusion process of I−/I3− in the electrolyte (low frequency region).69,70 Since an identical counter electrode was used for both DSSCs, the arc observed in the high frequency region is identical and therefore ignored. So main emphasis is given to the arc located in the middle frequency region. The larger semicircle for the ZnTPP–LC complex as compared to the LC device suggests that the charge recombination resistance (Rrec) is higher for the supramolecular dyad as compared the organic dye. This is consistent with the lower dark current value for the DSSC sensitized by ZnTPP–LC as well. This also indicates that the back electron recombination with the I3− form of the electrolyte is suppressed in the DSSC based on the ZnTPP–LC complex.
 | ||
| Fig. 6 (a) Nyquist and (b) Bode phase plots from EIS under dark conditions measured at a bias voltage of 0.65 V for DSSCs. | ||
The Bode phase plots of the DSSCs are shown in Fig. 6b. The peak frequency values (fmax) in the middle frequency region represent the electron transfer processes at the TiO2/dye/electrolyte interface, and were found to be 7.12 Hz and 4.54 Hz, for DSSCs based on LC and ZnTPP–LC dyes respectively. As it is known by the literature, fmax is associated with the electron lifetime (τe) according to the relation,71 i.e. τe = 1/2πfmax. The values of τe for the DSSCs based on LC and ZnTPP–LC are 22 ms and 36 ms, respectively. The lower value of τe for the ZnTPP–LC based DSSC as compared to the LC based one, indicates a reduced rate of electron recombination. The higher value of Rrec and the lower value of τe for the solar cell based on ZnTPP–LC, result in an improved overall PCE value for the cell. Moreover, the adsorption of the LC moiety anchored onto the TiO2 surface, in the supramolecular DSSC, forms an insulating layer that effectively prevents back electron transfer from the semiconductor to the I3− form of the electrolyte leading to higher Voc and Jsc values, resulting to an overall enhancement in the cell's efficiency.
The Nyquist plots of EIS under illumination also give important information about the charge transport at the TiO2/dye/electrolyte interface. We have measured the EIS under illumination of the DSSCs at a forward bias equivalent to the open circuit voltage of each corresponding DSSC. From their Nyquist plots, the corresponding electron charge transfer resistance values (Rtr) were estimated and are compiled in Table 5. The Rtr value of the cell sensitized by ZnTPP–LC is lower than that for the other DSSC. Both Rtr and Rrec values of a DSSC are related to the τe and electron transport time τd (which is a measure of average time taken by the injected electron to FTO electrode) according to the following relation72 τd/τe = Rtr/Rrec.
| Compound | Rrec (Ω) | Rtr (Ω) | τe (ms) | τd (ms) | ηcc |
|---|---|---|---|---|---|
| LC | 45 | 34 | 22 | 16.22 | 0.57 |
| ZnTPP–LC | 61 | 21 | 35 | 12.05 | 0.74 |
The values of Rrec, Rtr, τe and τd are displayed in Table 5. The τd value is lower for DSSC based on our ZnTPP–LC supramolecular dyad than the other one. This fact indicates that a faster transport of the injected electrons towards FTO electrode takes place. The faster τd value is associated with its higher Jsc value and indicates that the electrons are collected onto FTO at a faster rate.
In addition, the Jsc value of the supramolecular DSSC also depends on its charge collection efficiency ηcc, which is given by the following equation: ηcc = (1 + τd/τe)−1.73 The higher value of ηcc for the DSSC sensitized by the ZnTPP–LC complex can ultimately be the reason for such an improved PCE value.
Conclusions
In this work, we have synthesized a novel organic dye (LC), which is a π-conjugated oPPV moiety that contains a cyanoacrylic acid and a pyridyl group, as a promising sensitizer for DSSCs. The photophysical and electrochemical measurements of LC reveal that it has suitable energy levels for efficient electron injection and regeneration processes, when used as a sensitizer for DSSCs. We also demonstrated the formation of a supramolecular dyad (ZnTPP–LC) via a metal–ligand bond, between the nitrogen atom of LC's pyridyl group and ZnTPP's metal center. Theoretical calculations revealed that there is negligible electronic interaction between the two constituent units (LC and ZnTPP) in the ground state of our supramolecular dyad and also the dyad possesses frontier orbital energy levels which are suitable for use as sensitizers in DSSCs. The organic solar cell based on LC, as well as the supramolecular DSSC based on ZnTPP–LC, were both fabricated and reached PCE values of 2.45 and 5.27% respectively. As demonstrated by the J–V curves, the IPCE and EI spectra of the two DSSCs, the higher PCE value of the supramolecular one can be attributed to its enhanced short circuit current (Jsc) under illumination, its longer electron lifetime (τe) and more effective charge recombination resistance between the injected electrons and the electrolyte.References
- M. Gratzel, Nature, 1991, 349, 740–741 CrossRef PubMed.
- B. Oregan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS PubMed.
- T. W. Hamann and J. W. Ondersma, Energy Environ. Sci., 2011, 4, 370–381 CAS.
- K. L. Wu, S. T. Ho, C. C. Chou, Y. C. Chang, H. A. Pan, Y. Chi and P. T. Chou, Angew. Chem., Int. Ed., 2012, 51, 5642–5646 CrossRef CAS PubMed.
- S. H. Jiang, X. F. Lu, G. Zhou and Z. S. Wang, Chem. Commun., 2013, 49, 3899–3901 RSC.
- Q. Y. Feng, X. W. Jia, G. Zhou and Z. S. Wang, Chem. Commun., 2013, 49, 7445–7447 RSC.
- S. F. Zhang, X. D. Yang, Y. H. Numata and L. Y. Han, Energy Environ. Sci., 2013, 6, 1443–1464 CAS.
- M. Graetzel, R. A. J. Janssen, D. B. Mitzi and E. H. Sargent, Nature, 2012, 488, 304–312 CrossRef CAS PubMed.
- M. Gratzel, Acc. Chem. Res., 2009, 42, 1788–1798 CrossRef CAS PubMed.
- A. Hagfeldt, G. Boschloo, L. C. Sun, L. Kloo and H. Pettersson, Chem. Rev., 2010, 110, 6595–6663 CrossRef CAS PubMed.
- M. Gratzel, J. Photochem. Photobiol., A, 2004, 164, 3–14 CrossRef CAS PubMed.
- M. K. Nazeeruddin, P. Pechy, T. Renouard, S. M. Zakeeruddin, R. Humphry-Baker, P. Comte, P. Liska, L. Cevey, E. Costa, V. Shklover, L. Spiccia, G. B. Deacon, C. A. Bignozzi and M. Gratzel, J. Am. Chem. Soc., 2001, 123, 1613–1624 CrossRef CAS PubMed.
- S. Hwang, J. H. Lee, C. Park, H. Lee, C. Kim, C. Park, M. H. Lee, W. Lee, J. Park, K. Kim, N. G. Park and C. Kim, Chem. Commun., 2007, 4887–4889 RSC.
- S. Ahmad, E. Guillen, L. Kavan, M. Gratzel and M. K. Nazeeruddin, Energy Environ. Sci., 2013, 6, 3439–3466 CAS.
- C. C. Chou, F. C. Hu, H. H. Yeh, H. P. Wu, Y. Chi, J. N. Clifford, E. Palomares, S. H. Liu, P. T. Chou and G. H. Lee, Angew. Chem., Int. Ed., 2014, 53, 178–183 CrossRef CAS PubMed.
- S. K. Das, B. Y. Song, A. Mahler, V. N. Nesterov, A. K. Wilson, O. Ito and F. D'Souza, J. Phys. Chem. C, 2014, 118, 3994–4006 CAS.
- W. M. Campbell, K. W. Jolley, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. Schmidt-Mende, M. K. Nazeeruddin, Q. Wang, M. Gratzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 11760–11762 CAS.
- J. F. Lu, X. B. Xu, K. Cao, J. Cui, Y. B. Zhang, Y. Shen, X. B. Shi, L. S. Liao, Y. B. Cheng and M. K. Wang, J. Mater. Chem. A, 2013, 1, 10008–10015 CAS.
- Y. Q. Wang, L. Xu, X. D. Wei, X. Li, H. Agren, W. J. Wu and Y. S. Xie, New J. Chem., 2014, 38, 3227–3235 RSC.
- G. E. Zervaki, M. S. Roy, M. K. Panda, P. A. Angaridis, E. Chrissos, G. D. Sharma and A. G. Coutsolelos, Inorg. Chem., 2013, 52, 9813–9825 CrossRef CAS PubMed.
- W. H. Li, L. P. Si, Z. H. Liu, Z. X. Zhao, H. S. He, K. Zhu, B. Moore and Y. B. Cheng, J. Mater. Chem. A, 2014, 2, 13667–13674 CAS.
- M. Urbani, M. Grätzel, M. K. Nazeeruddin and T. Torres, Chem. Rev., 2014, 114, 12330–12396 CrossRef CAS PubMed.
- T. Higashino and H. Imahori, Dalton Trans., 2015, 44, 448–463 RSC.
- L.-L. Li and E. W.-G. Diau, Chem. Soc. Rev., 2013, 42, 291–304 RSC.
- T. Bessho, S. M. Zakeeruddin, C. Y. Yeh, E. W. G. Diau and M. Gratzel, Angew. Chem., Int. Ed., 2010, 49, 6646–6649 CrossRef CAS PubMed.
- A. Yella, H. W. Lee, H. N. Tsao, C. Y. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. G. Diau, C. Y. Yeh, S. M. Zakeeruddin and M. Gratzel, Science, 2011, 334, 629–634 CrossRef CAS PubMed.
- C. L. Wang, C. M. Lan, S. H. Hong, Y. F. Wang, T. Y. Pan, C. W. Chang, H. H. Kuo, M. Y. Kuo, E. W. G. Diau and C. Y. Lin, Energy Environ. Sci., 2012, 5, 6933–6940 CAS.
- C. L. Wang, Y. C. Chang, C. M. Lan, C. F. Lo, E. W. G. Diau and C. Y. Lin, Energy Environ. Sci., 2011, 4, 1788–1795 CAS.
- Y. C. Chang, C. L. Wang, T. Y. Pan, S. H. Hong, C. M. Lan, H. H. Kuo, C. F. Lo, H. Y. Hsu, C. Y. Lin and E. W. G. Diau, Chem. Commun., 2011, 47, 8910–8912 RSC.
- S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Gratzel, Nat. Chem., 2014, 6, 242–247 CrossRef CAS PubMed.
- V. Nikolaou, K. Karikis, Y. Farre, G. Charalambidis, F. Odobel and A. G. Coutsolelos, Dalton Trans., 2015, 44, 13473–13479 RSC.
- V. Nikolaou, P. A. Angaridis, G. Charalambidis, G. D. Sharma and A. G. Coutsolelos, Dalton Trans., 2015, 44, 1734–1747 RSC.
- G. E. Zervaki, V. Tsaka, A. Vatikioti, I. Georgakaki, V. Nikolaou, G. D. Sharma and A. G. Coutsolelos, Dalton Trans., 2015, 44, 13550–13564 RSC.
- Z. E. Galateia, N. Agapi, N. Vasilis, G. D. Sharma and C. G. Athanassios, J. Mater. Chem. C, 2015, 3, 5652–5664 RSC.
- G. D. Sharma, S. A. Siddiqui, A. Nikiforou, G. E. Zervaki, I. Georgakaki, K. Ladomenou and A. G. Coutsolelos, J. Mater. Chem. C, 2015, 3, 6209–6217 RSC.
- C. Stangel, A. Bagaki, P. A. Angaridis, G. Charalambidis, G. D. Sharma and A. G. Coutsolelos, Inorg. Chem., 2014, 53, 11871–11881 CrossRef CAS PubMed.
- C. Schubert, J. T. Margraf, T. Clark and D. M. Guldi, Chem. Soc. Rev., 2015, 44, 988–998 RSC.
- J. Warnan, F. Buchet, Y. Pellegrin, E. Blart and F. Odobel, Org. Lett., 2011, 13, 3944–3947 CrossRef CAS PubMed.
- N. K. Subbaiyan and F. D'Souza, J. Porphyrins Phthalocyanines, 2013, 17, 733–741 CrossRef CAS.
- J. Deisenhofer, O. Epp, K. Miki, R. Huber and H. Michel, Nature, 1985, 318, 618–624 CrossRef CAS PubMed.
- H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809–1818 CrossRef CAS PubMed.
- S. Gunes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324–1338 CrossRef PubMed.
- J. L. Segura, N. Martin and D. M. Guldi, Chem. Soc. Rev., 2005, 34, 31–47 RSC.
- B. C. Thompson and J. M. J. Frechet, Angew. Chem., Int. Ed., 2008, 47, 58–77 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. C, 2007, 111, 2834–2860 CAS.
- S. Fukuzumi, Phys. Chem. Chem. Phys., 2008, 10, 2283–2297 RSC.
- V. Balzani, A. Credi and M. Venturi, ChemSusChem, 2008, 1, 26–58 CrossRef CAS PubMed.
- H. Imahori and S. Fukuzumi, Adv. Funct. Mater., 2004, 14, 525–536 CrossRef CAS PubMed.
- L. M. Goncalves, V. D. Bermudez, H. A. Ribeiro and A. M. Mendes, Energy Environ. Sci., 2008, 1, 655–667 CAS.
- C. Stangel, C. Schubert, S. Kuhri, G. Rotas, J. T. Margraf, E. Regulska, T. Clark, T. Torres, N. Tagmatarchis, A. G. Coutsolelos and D. M. Guldi, Nanoscale, 2015, 7, 2597–2608 RSC.
- A. Kira, T. Umeyama, Y. Matano, K. Yoshida, S. Isoda, J. K. Park, D. Kim and H. Imahori, J. Am. Chem. Soc., 2009, 131, 3198–3200 CrossRef CAS PubMed.
- M. K. R. Fischer, I. Lopez-Duarte, M. M. Wienk, M. V. Martinez-Diaz, R. A. J. Janssen, P. Bauerle and T. Torres, J. Am. Chem. Soc., 2009, 131, 8669–8676 CrossRef CAS PubMed.
- N. K. Subbaiyan, L. Obraztsov, C. A. Wijesinghe, K. Tran, W. Kutner and F. D'Souza, J. Phys. Chem. C, 2009, 113, 8982–8989 CAS.
- F. D'Souza and O. Ito, Coord. Chem. Rev., 2005, 249, 1410–1422 CrossRef PubMed.
- R. Chitta and F. D'Souza, J. Mater. Chem., 2008, 18, 1440–1471 RSC.
- N. K. Subbaiyan, C. A. Wijesinghe and F. D'Souza, J. Am. Chem. Soc., 2009, 131, 14646–14647 CrossRef CAS PubMed.
- W. Kohn and L. J. Sam, Phys. Rev., 1965, 140, A1133 CrossRef.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Lyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, C. Gonzalez and J. A. Pople, Gaussian 03, rev. D.01, Gaussian, Inc., Wallingford CT, 2004 Search PubMed.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- M. Cossi, V. Barone, R. Cammi and J. Tomasi, Chem. Phys. Lett., 1996, 255, 327–335 CrossRef CAS.
- G. E. Zervaki, P. A. Angaridis, E. N. Koukaras, G. D. Sharma and A. G. Coutsolelos, Inorg. Chem. Front., 2014, 1, 256–270 RSC.
- J. Li, Name Reactions, Springer International Publishing, 2014, ch. 147, pp. 344–346 Search PubMed.
- P. Rothemund and A. R. Menotti, J. Am. Chem. Soc., 1948, 70, 1808–1812 CrossRef CAS.
- M. Liang and J. Chen, Chem. Soc. Rev., 2013, 42, 3453–3488 RSC.
- C. Y. Huang and Y. O. Su, Dalton Trans., 2010, 39, 8306–8312 RSC.
- J. Bisquert and V. S. Vikhrenko, J. Phys. Chem. B, 2004, 108, 2313–2322 CrossRef CAS.
- J. Bisquert, F. Fabregat-Santiago, I. Mora-Sero, G. Garcia-Belmonte and S. Gimenez, J. Phys. Chem. C, 2009, 113, 17278–17290 CAS.
- R. Kern, R. Sastrawan, J. Ferber, R. Stangl and J. Luther, Electrochim. Acta, 2002, 47, 4213–4225 CrossRef CAS.
- M. Adachi, M. Sakamoto, J. T. Jiu, Y. Ogata and S. Isoda, J. Phys. Chem. B, 2006, 110, 13872–13880 CrossRef CAS PubMed.
- F. Fabregat-Santiago, J. Bisquert, L. Cevey, P. Chen, M. K. Wang, S. M. Zakeeruddin and M. Gratzel, J. Am. Chem. Soc., 2009, 131, 558–562 CrossRef CAS PubMed.
- J. van de Lagemaat and A. J. Frank, J. Phys. Chem. B, 2000, 104, 4292–4294 CrossRef CAS.
- Q. Wang, Z. P. Zhang, S. M. Zakeeruddin and M. Gratzel, J. Phys. Chem. C, 2008, 112, 10585 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16394c |
| This journal is © The Royal Society of Chemistry 2015 |