Fulleropyrrolidine molecular dumbbells act as multi-electron-acceptor triads. Spectroscopic, electrochemical, computational and morphological characterizations†
Aleksandra Mitrovića,
Jelena Stevanovića,
Milos Milčića,
Andrijana Žekićb,
Dalibor Stankovićc,
Shigui Chend,
Jovica D. Badjićd,
Dragana Milića and
Veselin Maslak*a
aFaculty of Chemistry, University of Belgrade, Studentski trg 16, P. O. B. 51, 11158 Belgrade, Serbia. E-mail: vmaslak@chem.bg.ac.rs
bFaculty of Physics, University of Belgrade, Studentski trg 12, 11000 Belgrade, Serbia
cInnovation Center of the Faculty of Chemistry, Studentski trg 12, 11000 Belgrade, Serbia
dDepartment of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Avenue, Columbus, Ohio 43210, USA
First published on 12th October 2015
Abstract
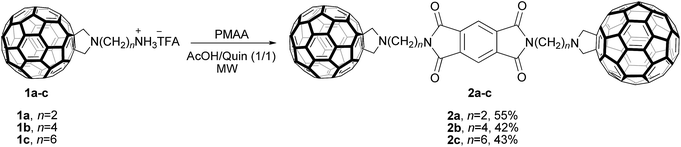
We synthesized three dumbbell-like compounds 2a–c, each containing two C60 groups at the periphery and pyromellitic diimide (PMDI) in the middle, and examined their electronic as well as assembly characteristics with both experimental and computational methods. Cyclic voltammetry (CV) measurements revealed that each of three electron-accepting (AAA) triads could accommodate up to eight electrons. Computational studies (density functional theory, DFT) of 2a–c at PBEPBE/6-311G(d,p) level of theory, with B3LYP/6-31G(d) optimized geometries, revealed that HOMO–LUMO energy gaps are similar to those of the model compound [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). Compounds 2a–c were also found to assemble into vesicles and nanoparticles on the copper grid (100–300 nm, TEM), while giving more sizeable aggregates after a deposition on the glass (SEM, >5 μm). Understanding the packing of 2a–c on various solid substrates, as well as the assembly characteristics in general, is important for tuning the properties and fabrication of electronic/optical devices. On the basis of the results of conformational analysis (MM and DFT calculations), we deduced that different alkyl spacers in 2a–c ought to play a role in π–π interactions between the aromatic components of the triad to guide the packing and therefore morphology of the material.
Introduction
In the pursuit of useful nano-electronic devices, scientists have incorporated electron-accepting fullerenes into a large number of hybrid structures.1–6 These systems are often composed of C60 conjugated to planar aromatics to generate molecules in which individual characteristics of the components are often modulated, to give rise to new properties.7 Platforms with one or two C60 groups, covalently attached to electroactive species, are labeled as fullero-dyads and fullero-triads. Importantly, different synthetic strategies have been developed for accessing architectures like C60–donor (AD), C60–acceptor (AA) or C60–donor–C60 (ADA). Within these structures, the electronic and electrochemical properties of C60 are modulated to allow energy and/or electron transfer between the sites.8–13 It is important to note that one of the highest power conversion efficiencies (PCE) in solution-processed organic photovoltaic cells is based on the polymer blends with fullerene derivative as the acceptor component.14,15Still, fullerene-building blocks are yet to become essential components of practical electrical conductors, photovoltaic cells, electroluminescence devices and field-effect transistors.16 It appears that the key challenge for constructing fullerene-based devices, having a desired performance, is in achieving a high level of control over the material's morphology at the nano scale.17 In line with it, directional noncovalent forces, such as hydrogen bonding and aromatic interactions, could enable a control of the self-assembly process for creating more ordered structures to perhaps permit the bottom-up engineering of fullerene-based devices.18–20
In accord with the discussion, dumbbell-like molecules (Fig. 1) consisting of two π-bridged fullerenes (C60–π system–C60) have been of an interest to both chemists and materials scientists. Due to the presence of two spheroid units, at the periphery, these triads are expected to have an improved solubility, such enabling more extensive examination and potential application. In addition, the fullerene groups can at periphery act as “molecular alligator clips” for anchoring to give a low spread of low-bias conductance.21 As examples of symmetric triads prepared recently, the following electron-donating units have been used to comprise the central core: oligomers (OPVs, OTVs, OPEs),22–25 triphenylamines (TPhAs),26 phenyl/fluorene,21 dithienosilole-dibenzothiadiazole (DTSDBT),27 tetrathiafulvalene (TTF),28 6b,10b-dihydrobenzo[j]cyclobut[a]acenaphthylene (DBCA).29 Interestingly, only few systems have been made to carry an electron-accepting central unit. In one instance, Champness, Khlobystov et al. examined the utility of the triad having 3,4,9,10-perylene tetracarboxylic diimide (PTCDI) group bridging two fullerenes. This system, with multiple redox sites, was capable of accommodating up to six electrons in a predictable and controllable manner.30 Naphtalenetetracarboxylic diimide (NTCDI), as another representative, have been employed to act as a component of fullerene-based rotaxanes.27 These compounds contained two C60 fullerenes, acting as stoppers at both termini, while the NTCDI unit would form noncovalent contacts with the rotaxane's macrocycle.27 In line with these efforts, we hereby describe a study of three symmetric triads 2a–c containing two fulleropyrrolidines covalently attached to pyromellitic diimide (PMDI) via alkyl chains (Scheme 1). We first prepared and characterized compounds 2a–c (C60–PMDI–C60, Scheme 1) and then examined their electronic, electrochemical and assembly characteristics.
Experimental section
Synthesis of materials
Compounds 2a–c are brown solids, stable at ambient conditions. These molecules were obtained in moderate yield 42–55%, by reacting pyromellitic acid anhydride (PMAA) with fulleropyrrolidine salts 1a–c31 (Scheme 1). To optimize reaction conditions, we varied solvent composition CH3CO2H/quinoline and adjusted microwave irradiation power and time (see Table S1†). In particular, a suspension of 1a–c (0.0052 mmol) and pyromellitic acid anhydride (0.0026 mmol) in CH3CO2H/quinoline = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture (1.2 mL) was irradiated in a microwave reactor for 30 min, with an inner temperature of 130 °C and applied pulse of 300 W. Crude product was precipitated by the addition of methanol and then purified by column chromatography (SiO2) with CH3CO2CH2CH3/toluene = 1/9 mixture as an eluent. Subsequent precipitation with methanol, from a highly concentrated solution in CHCl3, gave pure 2a–c as brown powders in 42–55% yields.
1 mixture (1.2 mL) was irradiated in a microwave reactor for 30 min, with an inner temperature of 130 °C and applied pulse of 300 W. Crude product was precipitated by the addition of methanol and then purified by column chromatography (SiO2) with CH3CO2CH2CH3/toluene = 1/9 mixture as an eluent. Subsequent precipitation with methanol, from a highly concentrated solution in CHCl3, gave pure 2a–c as brown powders in 42–55% yields.
![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) : 2930, 1719, 1370, 1093, 760, 481 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (4200), 704 (380). 1H (CDCl3, 500 MHz) δ 8.37 (s, 4H), 4.48 (s, 8H), 4.47 (s, 4H), 4.34–4.28 (m, 4H), 3.50–3.44 (m, 4H). 13C (CDCl3, 125 MHz) δ 166.2 (4C); 154.8 (Cf-12); 147.5 (Cf-17); 146.4 (Cf-7); 146.1 (Cf-11); 145.6 (Cf-16); 145.5 (Cf-5); 144.7 (Cf-9); 143.3 (Cf-15); 142.8 (Cf-8); 142.2 (Cf-6); 142.1 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.1 (Cf-10); 140.3 (Cf-3); 138.8 (4C), 136.2 (4C); 118.9 (2CH); 70.8 (4C); 67.5 (4CH2); 37.5 (2CH2); 33.8 (2CH2). MALDI/TOF: m/z calcd for [C138H18N4O4+H]+ 1796, measured 1796.
: 2930, 1719, 1370, 1093, 760, 481 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (4200), 704 (380). 1H (CDCl3, 500 MHz) δ 8.37 (s, 4H), 4.48 (s, 8H), 4.47 (s, 4H), 4.34–4.28 (m, 4H), 3.50–3.44 (m, 4H). 13C (CDCl3, 125 MHz) δ 166.2 (4C); 154.8 (Cf-12); 147.5 (Cf-17); 146.4 (Cf-7); 146.1 (Cf-11); 145.6 (Cf-16); 145.5 (Cf-5); 144.7 (Cf-9); 143.3 (Cf-15); 142.8 (Cf-8); 142.2 (Cf-6); 142.1 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.1 (Cf-10); 140.3 (Cf-3); 138.8 (4C), 136.2 (4C); 118.9 (2CH); 70.8 (4C); 67.5 (4CH2); 37.5 (2CH2); 33.8 (2CH2). MALDI/TOF: m/z calcd for [C138H18N4O4+H]+ 1796, measured 1796.![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) : 2925, 1719, 1368, 1118, 728, 526 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (5200), 704 (380). 1H (CDCl3, 500 MHz) δ 8.24 (s, 4H); 4.36 (s, 8H); 3.94 (t, 4H, J = 6.5); 3.13 (t, 4H, J = 7.5); 2.12–2.07 (m, 4H); 1.99–1.94 (m, 4H). 13C (CDCl3, 125 MHz): δ 165.6 (4C); 154.5 (Cf-12); 147.0 (Cf-17); 146.0 (Cf-7); 145.8 (Cf-11); 145.7 (Cf-16); 145.4 (Cf-5); 145.2 (Cf-9); 145.0 (Cf-15); 144.3 (Cf-8); 142.9 (Cf-6); 142.4 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.7 (Cf-10); 140.0 (Cf-3); 137.0 (4C); 136.0 (4C); 118.0 (2CH); 70.3 (4C); 67.7 (4CH2); 53.8 (2CH2); 38.3 (2CH2); 26.4 (2CH2); 26.1 (2CH2). MALDI/TOF: m/z calcd for [C142H26N4O4+H]+ 1853, measured 1853.
: 2925, 1719, 1368, 1118, 728, 526 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (5200), 704 (380). 1H (CDCl3, 500 MHz) δ 8.24 (s, 4H); 4.36 (s, 8H); 3.94 (t, 4H, J = 6.5); 3.13 (t, 4H, J = 7.5); 2.12–2.07 (m, 4H); 1.99–1.94 (m, 4H). 13C (CDCl3, 125 MHz): δ 165.6 (4C); 154.5 (Cf-12); 147.0 (Cf-17); 146.0 (Cf-7); 145.8 (Cf-11); 145.7 (Cf-16); 145.4 (Cf-5); 145.2 (Cf-9); 145.0 (Cf-15); 144.3 (Cf-8); 142.9 (Cf-6); 142.4 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.7 (Cf-10); 140.0 (Cf-3); 137.0 (4C); 136.0 (4C); 118.0 (2CH); 70.3 (4C); 67.7 (4CH2); 53.8 (2CH2); 38.3 (2CH2); 26.4 (2CH2); 26.1 (2CH2). MALDI/TOF: m/z calcd for [C142H26N4O4+H]+ 1853, measured 1853.![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) : 2933, 1721, 1365, 1118, 731, 525 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (6600), 704 (380). 1H (CDCl3, 500 MHz) δ 8.21 (s, 4H); 4.37 (s, 8H); 3.79 (t, 4H, J = 7); 3.07 (t, 4H, J = 7.5); 1.96–1.90 (m, 4H); 1.86–1.79 (m, 4H); 1.74–1.68 (m, 4H); 1.58–1.52 (m, 4H). 13C (CDCl3, 125 MHz) δ 165.6 (4C); 154.7 (Cf-12); 147.0 (Cf-17); 146.0 (Cf-7); 145.8 (Cf-11); 145.8 (Cf-16); 145.4 (Cf-5); 145.2 (Cf-9); 145.0 (Cf-15); 144.3 (Cf-8); 142.9 (Cf-6); 142.4 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.7 (Cf-10); 140.0 (Cf-3); 137.0 (4C); 136.0 (4C); 117.8 (2CH); 70.4 (4C); 67.7 (4CH2); 54.6 (2CH2); 38.4 (2CH2); 28.7 (2CH2); 28.5 (2CH2); 27.1 (2CH2); 26.8 (2CH2). MALDI/TOF: m/z calcd for [C146H34N4O4+H]+ 1909, measured 1909.
: 2933, 1721, 1365, 1118, 731, 525 cm−1. UV-VIS (CHCl3) λ (ε, M−1 cm−1): 430 (6600), 704 (380). 1H (CDCl3, 500 MHz) δ 8.21 (s, 4H); 4.37 (s, 8H); 3.79 (t, 4H, J = 7); 3.07 (t, 4H, J = 7.5); 1.96–1.90 (m, 4H); 1.86–1.79 (m, 4H); 1.74–1.68 (m, 4H); 1.58–1.52 (m, 4H). 13C (CDCl3, 125 MHz) δ 165.6 (4C); 154.7 (Cf-12); 147.0 (Cf-17); 146.0 (Cf-7); 145.8 (Cf-11); 145.8 (Cf-16); 145.4 (Cf-5); 145.2 (Cf-9); 145.0 (Cf-15); 144.3 (Cf-8); 142.9 (Cf-6); 142.4 (Cf-14); 142.0 (Cf-4); 141.8 (Cf-12,13); 141.7 (Cf-10); 140.0 (Cf-3); 137.0 (4C); 136.0 (4C); 117.8 (2CH); 70.4 (4C); 67.7 (4CH2); 54.6 (2CH2); 38.4 (2CH2); 28.7 (2CH2); 28.5 (2CH2); 27.1 (2CH2); 26.8 (2CH2). MALDI/TOF: m/z calcd for [C146H34N4O4+H]+ 1909, measured 1909.Computational details
In order to investigate π–π aromatic interactions between fullerene and PMDI moieties, we employed DFT calculations. It was shown previously32 that B97-D functional,33 with included Grimme's D2 dispersion correction, is the method of choice for computing π–π stacking interactions. This method was successfully applied for quantifying concave–convex interactions in corannulene34 and coronene35 dimers, fullerene interactions with corannulenes and sumanenes36 and fullerene interactions with molecular tweezers.37,38 The geometry of fullerene–PMDI complex was fully optimized with B97-D functional using triple zeta basis set (TZVP) from Ahlrichs and coworkers.39 Interaction energy (Eint) was calculated at the same level of theory (B97-D/TZVP) with included Boys and Bernardi counterpoise correction40 to account for basis set superposition error (BSSE). Deformation energy (Edef) was calculated as a difference between the energy of monomers in their optimal geometries and their geometries within the complex. The full complexation energy (Ecomplex) was obtained using the formula:| Ecomplex = Eint − Edef | (1) |
Due to the large size and conformational flexibility of 2a–c, detailed computational study was performed only on compound 2a containing two CH2 groups. The conformational search was performed with molecular mechanic force field implemented in AMMP program, using systematic search method with Vega ZZ (version 3.0) interface.41 The geometries of three most abundant conformers of compound 2a were fully optimized in gas phase with B3LYP functional and 6-31G(d) basis set with included Grimme's D2 dispersion correction. Energies of the conformers were calculated on B3LYP/6-31G(d) optimized geometries using same functional and dispersion correction, but with larger 6-311G(d,p) basis set in toluene solution. Solvent effect was simulated with SMD implicit solvation method.42 Non-Covalent Interactions (NCI) visualization index is calculated using NCI plot program.43 HOMO and LUMO orbital energies are calculated in gas phase, on B3LYP/6-31G(d) optimized geometries, using PBEPBE functional and relatively large basis set 6-311G(d,p). This method was recently proven to be quite reliable in calculating HOMO–LUMO energies and other molecular properties in fulleropyrrolidine44 and PCBM-like fullerene derivatives.45
Properties of the first excited state were investigated using TD-DFT technique, with B3LYP functional and 6-311G(d,p) basis set. The Fukui function and spin densities were calculated with B3LYP/6-311G(d,p) method by adding electron to B3LYP/6-31G(d) optimized geometries. All the orbitals were drown in gOpenMol46 program from HF/6-311G(d,p) wavefunction.
All the quantum chemical calculations were done in Gaussian 09 program package.47
Results and discussion
Optical properties
UV-vis spectra of 2a–c, dissolved in CHCl3, were recorded between 300 and 800 nm at a room temperature (Fig. 2). Optical absorptions are for all three compounds identical, with bands characteristic for fulleropyrrolidine derivatives. The UV region of 2a–c is dominated by the strong absorption, which corresponds to the electronic transitions with high oscillator strength from the ground state to the excited states (0 → *0), analogously to the parent C60. In the visible region, a shoulder at 430 nm is accompanied with a weak band around 700 nm. The signal at 430 nm indicates the presence of [6,6]-functionalized fullerene derivatives.48The UV-vis spectra of 2a–c appear to be a superposition of those corresponding to fulleropyrrolidine and PMDI components. The emission spectra of 2a–c (400–850 nm, Fig. S1†) were collected after excitation at 400 nm and are in good agreement with the absorption features. The *0 → 0 emission (λmax, 709 nm) and 0 → *0 absorption bands (λmax, 704 nm) differ very slightly to provide additional evidence for the absence of a charge transfer interaction between two chromophores.
Electrochemistry
The electrochemical characteristics of fullerene-based 2a–c were investigated with cyclic voltammetry in o-dichlorobenzene–DMF = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution mixture (Fig. 3). In order to understand the electron transfer pathways, CV data were collected for structurally similar reference compounds N,N-dihexylpyromellitic diimide (DHPMDI) and N-methylfulleropyrrolidine (NMFP).
1 solution mixture (Fig. 3). In order to understand the electron transfer pathways, CV data were collected for structurally similar reference compounds N,N-dihexylpyromellitic diimide (DHPMDI) and N-methylfulleropyrrolidine (NMFP).
 | ||
Fig. 3 Cyclic voltammogram (CV) of 2a at 50 mV s−1 rate in o-dichlorobenzene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF = 2 DMF = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture, containing 0.1 M nBu4NPF6. 1 mixture, containing 0.1 M nBu4NPF6. | ||
The half-wave potential values for all compounds are given in Table 1. In the potential range between 0 and −2.8 V five quasi-reversible reductions were observed for 2a and 2b, whereas 2c showed four reduction waves. For illustrative clarity, CV curves for 2b–c and reference compounds are omitted from Fig. 3 and can be found in Fig. S2.† As can be seen from Table 1, the half-wave potential values, associated with the first fullerene reduction, are cathodically shifted by 100–120 mV, compared to the parent C60. Indeed, a decrease in the electron activity of the functionalized fullerene is expected due to a partial loss in π-conjugation.49 In the second reduction process, one electron is accepted by the PMDI moiety: the half wave potential of −1.16 V for 2a (−1.13 V for 2b, −1.15 V for 2c) compared with DHPMDI (1.10 V) indicates the increase of electronic density on this electron-poor organic addend. Third E1/2 value can be assigned to fullerene cages due to its good correlation with second reduction peak of the model NMFP (−1.28 V, Table 1). As the reduction goes to more negative potentials, the assignment of the redox processes, based on a similarity to the corresponding model compounds, becomes less straightforward. We assign reduction peaks IV and V, to the second PMDI and third C60 reduction, respectively. For compound 2c, the last two reduction peaks could not be resolved by changing the scan rate of the experiment. A gradual broadening of the redox waves might be ascribed to the presence of longer and more flexible alkyl chains (six CH2 groups) causing a decrease in the rate constant characterizing electron transfer processes at the electrode surface.
In line with reductions of other related symmetric C60-dimers and triads,30,50 each of the fullerene reduction waves is in 2a–c commensurate with a two-electron uptake. The peak current of these reductions is thus higher than for those associated with the PMDI reductions, indicating that the latter correspond to a one-electron uptake.
Cyclic voltammograms of all three triads (Fig. S2†) show that C60 and PMDI moieties are accommodating electrons independently of each other. It should also be noted that no significant influence of the alkyl spacer on the electrochemical properties was found, except for the dumbbell 2c where a greater conformational flexibility of alkyl chains, perhaps, causes a poor resolution of the reduction peaks. As shown in Table 2, dumbbell-like 2a–c display a substantial electrochemical response with the ability of accommodating up to eight electrons to emphasize its possible use as an PCBM-like electron acceptor in polymer solar cells.
| Compd | Parameters | PBEPBE/6-311G(d,p)a |
|---|---|---|
| a Cited from ref. 44.b Extended conformer.c Single-folded conformer.d Double-folded conformer. | ||
| C60 | HOMO/eV | −5.867 |
| LUMO/eV | −4.181 | |
| Egap/eV | 1.686 | |
| PCBM | HOMO/eV | −5.522 |
| LUMO/eV | −4.006 | |
| Egap/eV | 1.516 | |
| 2a | HOMO/eV | −5.575b, −5.447c, −5.417d |
| LUMO/eV | −4.234b, −4.238c, −4.257d | |
| Egap/eV | 1.341b, 1.209c, 1.16d | |
DFT calculations
The Wudl group has extensively studied electronic properties of spiromethanofullerene with fluorenyl planar framework being perpendicular to the surface of C60.51,52 They concluded that this topological arrangement is responsible for ‘though-space’ interactions between fluorenyl moiety and C60 causing the first reduction potential to become less negative than the same one in parent C60. This prompted us to investigate possible concave/convex π–π aromatic interactions between electron accepting organic addends (C60 and PMDI) within 2a–c and to study whether their spatial arrangement has a measurable effect on the electronic and self-assembly properties of these compounds.A π–π aromatic interaction between fullerene and PMDI (as a model complex) were investigated computationally with B97-D/TZVP method. In the optimized geometry of fullerene–PMDI complex (Fig. 4a) a PMDI molecule has adopted a concave geometry with 8.0° deviation from planarity. This bending of the PMDI molecule allows a greater contact surface between molecules which could give rise to stronger π–π interactions.35 The calculated complexation energy (Ecomplex) is 9.76 kcal mol−1. This complexation energy is relatively small and only half the size of the complexation energy of fullerene with corannulene (17.03 kcal mol−1) and sumanene (18.47 kcal mol−1) aromatics, both well known as concave hosts for fullerene. Despite, the obtained values indicate a possibility for noncovalent intermolecular interactions in compounds 2a–c, especially in polar non-aromatic solvents.
The nature of the interaction was further investigated using non-covalent interactions (NCI) visualization index.46 The NCI index, calculated for the fullerene–PMDI complex (Fig. 4b), reveals a large area of weak and attractive π–π contacts as a primary source of the interaction between these two molecules.
Conformational search for stable conformers of compound 2a produced three clusters of conformers (Fig. 5). In the first cluster (Fig. 5a) were the structures with both fullerene alkyl arms fully extended away from the PMDI part with no intramolecular π–π contacts. In the conformers of the second cluster (Fig. 5c), one fullerene arm is folded toward the PMDI part and in the third cluster both fullerene arms are folded towards PMDI part of the molecules (Fig. 5b). One representative structure of each cluster was additionally optimized and structures of extended, single-folded and double-folded conformers were obtained (Fig. 5a–c). The potential energies of all three conformers of compound 2a in toluene solution are evaluated at B3LYP/6-311G(d,p) level of theory. The most stabile conformation is single-folded (Fig. 5c), while double-folded (Fig. 5b) and extended (Fig. 5a) conformations are somewhat less stable (1.1 kcal mol−1 and 4.1 kcal mol−1, respectively).
It is worth noting that entropic factors are not included in our analysis. The entropy could, perhaps, significantly contribute to the stability of conformers, and especially in the solvent phase.
The second part of our computational investigation was focused on understanding the electronic properties of compound 2a. HOMO–LUMO energy gaps were calculated for the extended, single-folded (Fig. 5b) and double-folded (Fig. 5c) conformers. Obtained results were compared to the appropriate values for the reference C60 and PCBM. Summarized data are shown in Table 2. It can be seen that HOMO–LUMO energy gap is 0.34–0.52 eV lower for all conformers of compound 2a when compared to C60. Concurrently, both HOMO (0.29–0.45 eV) and LUMO energies (0.08–0.05 eV) are more negative for three conformers of 2a. On the other hand HOMO/LUMO energies and Egap of all 2a conformers more closely reassemble those of the PCBM acceptor (Table 2). All conformers of 2a display higher-lying LUMO and reduced bandgap which is very important in terms of improving the open-circuit voltage Voc and light harvesting capacity of the acceptor component in BHJ solar cells.53 Our further investigation of electron accepting features of compound 2a was oriented towards calculating the properties of excited state. TD-DFT calculations at B3LYP/6-311G(d,p) level of theory was employed in order to investigate the properties of first excited state. TD-DFT calculations predicts that first energy transitions will occur at energies of 1.881, 1.860 and 1.873 eV for extended, single folded and double folded conformer, respectively. The electron density difference maps between first excited and ground state are shown in Fig. 6. For the extended and single folded conformation there is no charge transfer between fullerene and PMDI parts of the molecule, indicating attenuated potential for the OPV n-phase material; excitation is localized on the fullerene cage (Fig. 6a and b). Due to favorable overlap of orbitals, a little of the electron transfer from fullerene to PMDI is observed in the double folded conformation (Fig. 6c).
Similarly, the Fukui function and Mulliken spin densities calculations (see ESI Fig. S3 and S4†) have shown that fullerene cage will be the most probable target of nucleophile attack. For radical monoanionic form of compound 2a, from 75% to 90% of additional electron spin density is located at the two fullerene cages.
Morphology characterization
The aggregation behavior of compounds 2a–c was investigated with scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Samples were prepared by drop-drying process, akin to earlier procedures used in studying the assembly of C60.54 The aggregation of fullerene derivatives has proven to be an efficient approach toward creating remarkable architectures like: 1D fibers, 2D sheets, 3D spheres and flower-like objects.18,55–59 These interesting structures exist as a direct consequence of the three-dimensional shape of C60 undergoing aromatic interactions. In addition, different functional groups at the surface of fullerenes and the choice of solvents determine the final outcome of self-assembly process.To investigate the effect of the medium polarity on the aggregation of 2a–c, we used the following solvents: toluene, o-dichlorobenzene (ODCB) and chloroform as well as their 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixtures with 1,4-dioxane and iso-propanol. All samples were prepared in the following manner prior to SEM/TEM imaging. Compounds 2a–c were dissolved (1 mg) in 1 mL of solvent (∼0.5 μM) and a drop of the solution was deposited onto a glass-plate (SEM). Under toluene atmosphere, the solvent was allowed to evaporate during 48 h at a room temperature (SEM). For TEM measurement, 0.25 mM solution of 2a–c was deposited on a copper grid and subjected to drying under ambient conditions.
1 mixtures with 1,4-dioxane and iso-propanol. All samples were prepared in the following manner prior to SEM/TEM imaging. Compounds 2a–c were dissolved (1 mg) in 1 mL of solvent (∼0.5 μM) and a drop of the solution was deposited onto a glass-plate (SEM). Under toluene atmosphere, the solvent was allowed to evaporate during 48 h at a room temperature (SEM). For TEM measurement, 0.25 mM solution of 2a–c was deposited on a copper grid and subjected to drying under ambient conditions.
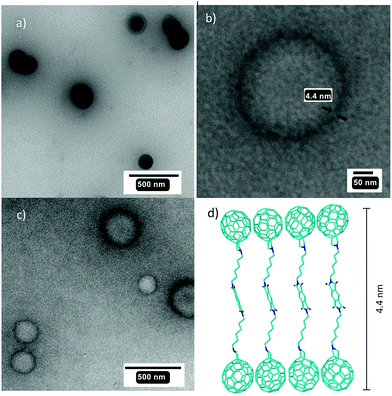
Different modes of assembly of 2a–c were observed by SEM and TEM measurements (Fig. 7 and 8). The apparent discrepancy resulted from different conditions used in the preparation of samples. The aggregation of molecules ought to be a function of the solid substrates on which it took place, the concentration of molecules and drying conditions (time, temperature, etc.). Compound 2a assembled into nanoparticles (Fig. 7a), while 2b and 2c (only 2c shown in Fig. 7b) showed a tendency to form unilamellar vesicles. The sizes of the vesicles were generally uniform with 200–300 nm in diameter. By a closer inspection of the single vesicle (HR TEM) we estimated the thickness of its membrane to be 4.4 nm. The width corresponds well to the length of the extended conformer of 2c (Fig. 7c). As presented in Fig. 8, compound 2a dissolved in toluene/dioxane (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) assembles into fiber containing sheets (or bundles) while in the same solvent mixture 2b aggregates into smaller hank-like objects (Fig. 8c). When processed from toluene/iPr-OH (2/1) 2a exhibits a flower-like structure (Fig. 8b), whereas 2b assembles into sheet fibers (Fig. 8d).
1) assembles into fiber containing sheets (or bundles) while in the same solvent mixture 2b aggregates into smaller hank-like objects (Fig. 8c). When processed from toluene/iPr-OH (2/1) 2a exhibits a flower-like structure (Fig. 8b), whereas 2b assembles into sheet fibers (Fig. 8d).
 | ||
| Fig. 8 SEM images of 2a prepared from (a) toluene/dioxane, (b) toluene/iPr-OH; 2b prepared from (c) toluene/dioxane, (d) toluene/iPr-OH and 2c prepared from (e) CHCl3/iPr-OH, (f) CHCl3/dioxane. | ||
When dissolved in CHCl3/iPr-OH (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture compound 2c gave micron-size clusters (Fig. 8e), whereas in CHCl3/dioxane (2/1) mixture “necklace-like” chains (Fig. 8f). In other solvent combinations compounds 2a–c showed various self-organized architectures with the corresponding images presented in ESI (Fig. S5†).
1) mixture compound 2c gave micron-size clusters (Fig. 8e), whereas in CHCl3/dioxane (2/1) mixture “necklace-like” chains (Fig. 8f). In other solvent combinations compounds 2a–c showed various self-organized architectures with the corresponding images presented in ESI (Fig. S5†).
To summarize, compounds 2a–c have a tendency to organize into nano- and micro-sized structures as a function of the length of their alkyl chains and experimental conditions. Importantly, the length of the extended conformer 2c bearing a hexyl chain is consistent with the thickness of the vesicle wall. This implies that the monolayer structure is in vesicles held by π–π (C60/C60, PMDI/PMDI) and van der Waals (CH2/CH2) interactions. With a large number of C60 exposed on the vesicle surface, “active cites” are created for fullerene–fullerene attraction between different vesicles, which could play a role in the observed hierarchical assembly by SEM.
We presume that compounds 2a and 2b, having shorter alkyl chains, give rise to weaker noncovalent contacts with a smaller solvophobic bias to lead to the formation of different structures, indicating a way for controlling the ordering of this type of supramolecular soft material.
Conclusions
In conclusion, we have successfully synthesized a series of fulleropyrrolidine molecular dumbbells (2a–c). Their electronic, electrochemical and self-assembly characteristics were determined with UV-vis/emission spectroscopies, cyclic voltammetry, DFT calculation, scanning electron and transmission electron microscopies. CV measurements confirmed that the functionalization of C60 preserved its electron-accepting characteristics. Reduction potentials are in line with values measured for the model NMFP compound, and therefore consistent with the notion that there is only a weak electronic coupling between the triad units. DFT calculations suggested that these weak interactions consist of concave/convex π–π contacts between C60 moieties and PMDI. Different modes of supramolecular assembly were observed for 2a–c. On the copper surface, compound 2a with a shorter alkyl chain connecting its building units assembled into nanoparticles (2a). Compounds 2b and 2c, with longer alkyl groups, formed nanosized vesicles. On a glass surface, however, 2a–c gave rise to larger (microsized) aggregates via, perhaps, a hierarchical ordering of nanoparticles and vesicles. The formation of supramolecular architectures appeared to be less dependent on the choice of the solvent and more influenced by the molecular design and the nature of supporting substrate yet other factors could also play a role. Our groups are currently examining the electronic and assembly characteristics of C60/PMDI triads containing stiffer and electron conjugated/non-conjugated linking groups on which we plan to report in future.Acknowledgements
Financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia is acknowledged (Project No. 172002). An experimental portion of this work (TEM measurements) was financially supported with funds obtained from the U.S. National Science Foundation (CHE-1305179) to JDB.Notes and references
- J. Roncali, Chem. Soc. Rev., 2005, 34, 483–495 RSC.
- T. Nishizawa, K. Tajima and K. Hashimoto, J. Mater. Chem., 2007, 17, 2440–2445 RSC.
- Y. Shirai, J.-F. Morin, T. Sasaki, J. M. Guerrero and J. M. Tour, Chem. Soc. Rev., 2006, 35, 1043–1055 RSC.
- K. Prassides, M. Keshavarz-K, J. C. Hummelen, W. Andreoni, P. Giannozzi, E. Beer, C. Bellavia, L. Cristofolini, R. Gonzalez, A. Lappas, Y. Murata, M. Malecki, V. I. Srdanov and F. Wudl, Science, 1996, 271, 1833 CAS.
- S. Yoshimoto, E. Tsutsumi, R. Narita, Y. Murata, M. Murata, K. Fujiwara, K. Komatsu, O. Ito and K. Itaya, J. Am. Chem. Soc., 2007, 129, 4366 CrossRef CAS PubMed.
- A. Mateo-Alonso, D. M. Guldi, F. Paolucci and M. Prato, Angew. Chem., Int. Ed., 2007, 46, 8120–8126 CrossRef CAS PubMed.
- G. Mehtaa and V. Singh, Chem. Soc. Rev., 2002, 31, 324–334 RSC.
- N. Martın, L. Sanchez, M. A. Herranz and D. M. Guldi, J. Phys. Chem. A, 2000, 104, 4648–4657 CrossRef.
- A. S. Konev, A. F. Khlebnikov, T. G. Nikiforova, A. A. Virtsev and H. Frauendorf, J. Org. Chem., 2013, 78, 2542–2552 CrossRef CAS PubMed.
- J. Baffreau, S. Leroy-Lhez, N. van Anh, R. M. Williams and P. Hudhomme, Chem.–Eur. J., 2008, 14, 4974–4992 CrossRef CAS PubMed.
- J. Baffreau, L. Ordronneau, S. Leroy-Lhez and P. Hudhomme, J. Org. Chem., 2008, 73, 6142–6147 CrossRef CAS PubMed.
- J. L. Segura and N. Martín, Chem. Soc. Rev., 2000, 29, 13–25 RSC.
- N. Martın, L. Sanchez, B. Illescas and I. Perez, Chem. Rev., 1998, 98, 2527–2547 CrossRef PubMed.
- K. L. J. Y. KIm, N. E. Coates, D. Moses, T. Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222 CrossRef PubMed.
- G. Li, V. Shrotriya, J. S. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CrossRef CAS PubMed.
- D. M. Guldi, B. M. Illescas, C. M. Atienza, M. Wielopolskia and N. Martın, Chem. Soc. Rev., 2009, 38, 1587–1597 RSC.
- S. Santhosh, B. H. Mohwald and T. Nakanishi, Chem. Soc. Rev., 2010, 39, 4021–4035 RSC.
- T. Nakanishi, Chem. Commun., 2010, 46, 3425–3436 RSC.
- D. M. Guldi and N. Martın, J. Mater. Chem., 2002, 12, 1978–1992 RSC.
- T. Wang, A. J. Pearson and D. G. Lidzey, J. Mater. Chem. C, 2013, 1, 7266–7293 RSC.
- J. K. Sørensen, J. Fock, A. H. Pedersen, A. B. Petersen, K. Jennum, K. Bechgaard, K. Kilsa, V. Geskin, J. Cornil, T. Bjørnholm and M. B. Nielsen, J. Org. Chem., 2011, 76, 245–263 CrossRef PubMed.
- M. J. Gomez-Escalonilla, F. Langa, J.-M. Rueff, L. Oswald and J.-F. Nierengarten, Tetrahedron Lett., 2002, 43, 7507–7511 CrossRef CAS.
- N. Zhou and Y. Zhao, J. Org. Chem., 2010, 75, 1499 Search PubMed.
- C. Atienza, B. Insuasty, C. Seoane, N. Martın, J. Ramey, G. M. A. Rahman and D. M. Guldi, J. Mater. Chem., 2005, 15, 124–132 RSC.
- K. Komatsu, N. Takimoto, Y. Murata, T. S. M. Wan and T. Wong, Tetrahedron Lett., 1996, 37, 6153–6156 CrossRef CAS.
- R. Zalesny, O. Loboda, K. Iliopoulos, G. Chatzikyriakos, S. Couris, G. Rotas, N. Tagmatarchis, A. Avramopoulos and M. Papadopoulos, Phys. Chem. Chem. Phys., 2010, 12, 373–381 RSC.
- Y. Nakamura, S. Minami, K. Iizuka and J. Nishimura, Angew. Chem., Int. Ed., 2003, 42, 3158–3162 CrossRef CAS PubMed.
- J. L. Segura, E. M. Priego, N. Martın, C. Luo and D. M. Guldi, Org. Lett., 2000, 2, 4021–4024 CrossRef CAS PubMed.
- A. Sastre-Santos, C. Parejo, L. Martın-Gomis, K. Ohkubo, F. Fernandez-Lazaro and S. Fukuzumi, J. Mater. Chem., 2011, 21, 1509–1515 RSC.
- T. W. Chamberlain, E. S. Davies, A. N. Khlobystov and N. R. Champness, Chem.–Eur. J., 2011, 17, 3759–3767 CrossRef CAS PubMed.
- A. Mitrovic, N. Todorovic, A. Zekic, D. Stankovic, D. Milic and V. Maslak, Eur. J. Org. Chem., 2013, 2188–2193 CrossRef CAS PubMed.
- D. Josa, J. Rodríguez-Otero, E. M. Cabaleiro-Lago and M. Rellán-Piñeiro, Chem. Phys. Lett., 2013, 557, 170–175 CrossRef CAS PubMed.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- T. Janowski, P. Pulay, A. A. S. Karunarathna, A. Sygula and S. Saebø, Chem. Phys. Lett., 2011, 512, 155–160 CrossRef CAS PubMed.
- M. R. Kennedy, L. A. Burns and C. D. Sherrill, J. Phys. Chem. A, 2012, 116, 11920–11926 CrossRef CAS PubMed.
- D. Josa, J. Rodrıguez-Otero, E. M. Cabaleiro-Lago, L. A. Santos and T. C. Ramalho, J. Phys. Chem. A, 2014, 118, 9521–9528 CrossRef CAS PubMed.
- C. Muck-Lichtenfeld, S. Grimme, L. Kobrynb and A. Sygula, Phys. Chem. Chem. Phys., 2010, 12, 7091–7097 RSC.
- D. Josa, J. Rodrıguez-Otero and E. M. Cabaleiro-Lago, Phys. Chem. Chem. Phys., 2015, 17, 13206 RSC.
- A. Schafer, C. Huberund and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS PubMed.
- A. Pedretti, L. Villa and G. Vistoli, J. Comput.-Aided Mol. Des., 2004, 18, 167–173 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
- E. R. Johnson, S. Keinan, P. Mori-Sánchez, J. Contreras-García, A. J. Cohen and W. Yang, J. Am. Chem. Soc., 2010, 132, 6498–6506 CrossRef CAS PubMed.
- X. Zhang, X. D. Li, L. X. Maa and B. Zhanga, RSC Adv., 2014, 4, 60342–60348 RSC.
- H. Wang, Y. He, Y. Li and H. Su, J. Phys. Chem. A, 2012, 116, 255–262 CrossRef CAS PubMed.
- L. Laaksonen, J. Mol. Graphics, 1992, 10, 33–34 CrossRef CAS.
- G. W. T. M. J. Frisch, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford CT, 2013 Search PubMed.
- M. Prato and D. M. Guldi, Acc. Chem. Res., 2000, 33, 695–703 CrossRef PubMed.
- T. Suzuki, Y. Maruyama, T. Akasaba, W. Ando, K. Kobayashi and S. Nagase, J. Am. Chem. Soc., 1994, 116, 1359–1363 CrossRef CAS.
- P. Timmerman, L. E. Witschel, F. Diederich, C. Boudon, J.-P. Gisselbrecht and M. Gross, Helv. Chim. Acta, 1996, 79, 6–20 CrossRef CAS PubMed.
- M. Eiermann, R. Haddon, B. Knigh, Q. Li, M. Maggini, N. Martin, T. Ohno, M. Prato, T. Suzuki and F. Wudl, Angew. Chem., Int. Ed., 1995, 34, 1591–1594 CrossRef CAS PubMed.
- T. Ohno, N. Martin, B. Knigh, F. Wudl, T. Suzuki and H. Yu, J. Org. Chem., 1996, 61, 1306–1309 CrossRef CAS.
- J. W. Ryan and Y. Matsuo, Sci. Rep., 2015, 5, 1–5 Search PubMed.
- C. Park, H. J. Song and H. C. Choi, Chem. Commun., 2009, 4803–4805 RSC.
- F. T. Edelmann, Angew. Chem., Int. Ed., 1999, 38, 1381–1387 CrossRef CAS.
- N. Martin, Chem. Commun., 2006, 2093–2104 RSC.
- E.-Y. Zhang and C.-R. Wang, Curr. Opin. Colloid Interface Sci., 2009, 14, 148–156 CrossRef CAS PubMed.
- N. Zhou, F. Erika, S. Merschrod and Y. Zhao, J. Am. Chem. Soc., 2005, 127, 14154–14155 CrossRef CAS PubMed.
- T. Nakanishi, K. Ariga, T. Michinobu, K. Yoshida, H. Takahashi, T. Teranishi, H. Mohwald and D. G. Kurth, Small, 2007, 3, 2019–2023 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Scheme S1; scanned NMR spectra of 2a–c; Tables S1 and S2; Fig. S1–S6. See DOI: 10.1039/c5ra16309a |
| This journal is © The Royal Society of Chemistry 2015 |