High performance of MoS2 microflowers with a water-based binder as an anode for Na-ion batteries†
P. Ramesh Kumar,
Young Hwa Jung and
Do Kyung Kim*
Department of Materials Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 305-701, Republic of Korea. E-mail: dkkim@kaist.ac.kr
First published on 14th September 2015
Abstract
Na-ion batteries have risen as an alternative system to current Li-ion batteries due to the wide range of availability and low price of sodium resources. Here we report the binder effect on sodium storage properties of MoS2 microflowers with nano-sized petals which are prepared by a combination of a hydrothermal reaction and solid-state reaction. The electrochemical performance of MoS2 microflowers with different binders is evaluated against pure Na metal in a half-cell configuration through a conversion reaction. Especially, the electrode of MoS2 microflowers with a Na-alginate binder shows an excellent cycling stability, delivering a high discharge capacity of 595 mA h g−1 after 50 cycles. The MoS2 microflowers with the Na-alginate binder also exhibit high rate capability, retaining a capacity of 236 mA h g−1 at 10C without any carbonaceous materials. The improved electrochemical performance was mainly attributed to the synergetic effect of the morphology of the MoS2 microflowers and good adhesive capabilities of the alginate binder. Furthermore, we report a Na-ion fuel cell using the MoS2 microflower anode with Na3V2O2x(PO4)2F3−2x/C as a cathode material.
1. Introduction
Na-ion batteries have been a focus recently since sodium is very cheap and abundant on the earth's crust, which makes it the most advantageous element for battery applications after lithium.1–4 Compared to Li-ion batteries, Na-ion batteries still have many obstacles for commercialization, although researchers have proposed a number of electrode materials for Na-ion batteries.5–7 In particular, the negative electrode for Na-ion batteries is one of the major challenging issues, because graphite, the representative anode material for Li-ion batteries, shows low Na-ion intercalation/deintercalation capability.8 Pure Na metal also cannot be used because of dendrite formation while charging–discharging and low melting point which causes safety issues. Hence, researchers have proposed several new anode candidates such as Na2Ti3O7, Sb2O4, TiO2, Na2C8H4O4, and SnSb/C for Na-ion batteries.9–13Compared to other existing anode materials, metal sulfides possess high theoretical specific capacities and comparatively low cost. Molybdenum disulfide (MoS2) is a promising material which has the dichalcogenide structure, with the molybdenum atoms coordinated by six sulfur atoms.14 Most of all, the laminar MoS2 bonded through weak van der Waals forces can host metal cations.15 Thus, MoS2 has been studied as an intercalation host for lithium.16,17 Very large number of works on Li/MoS2 has been done, but very few papers were demonstrated as Na interaction host for Na-ion batteries. Park et al. presented the electrochemical behaviours of Na/MoS2 cells at each discharge depth through analysis of the crystallographic changes by employing ex situ X-ray diffraction (XRD) and transmission electron microscopy (TEM).18 David et al. showed that the MoS2/graphene composite paper delivered good Na cycling ability with a stable charge capacity of ∼230 mA h g−1 with 99% efficiency.19 Wang et al. reported MoS2/C nanospheres as a promising anode for high performance sodium-ion batteries due to high specific capacity (520 mA h g−1 at 0.1C).20 Xie et al. prepared MoS2/graphene composite by hydrothermal method as an anode material for Na-ion batteries. And they tried to understand the synergistic effect between layered sulfides and graphene using computational studies.21 some researchers have presented high-performance MoS2/graphene composites for Na-ion batteries and Na-ion pseudo-capacitors by different methods.22,23 Recently, Bang et al. demonstrated stable operation of a Na-ion battery by using the exfoliated MoS2 nanosheets as an intercalation anode with stable discharge capacity of 160 mA h g−1.24
In this work, we present the high capacity pure MoS2 microflowers with nanopetals as an attractive candidate for Na-ion battery negative electrode material. We have combined MoS2 microflowers with different binders to improve the interface between active materials and the current collector. MoS2 microflowers were prepared by using hydrothermal followed by calcination to obtain pure microflowers. The electrochemical response of the prepared nanoparticles was studied using cyclic voltammetry and galvanostatic charge–discharge measurements. These MoS2 microflowers with sodium alginate binder could exhibit promising electrochemical performance such as high reversible capacity, excellent cycling performance and good rate capability when compared to other binders like polyvinylidene fluoride (PVDF) and polyethylene oxide (PEO).
2. Experimental
2.1 Synthesis of MoS2 microflowers
First of all, (NH4)2MoS4 was prepared by a procedure similar to that previously reported.25 H2S was passed through the solution of ammonium hydroxide and ammonium hepta-molybdenum. The temperature rise to 60 °C, and H2S bubbling was stopped as soon as the reaction mixture colored was turn in to pink. The pink colour (NH4)2MoS4 solution was transferred to a 80 mL Teflon-lined autoclave and placed inside a muffle furnace at 180 °C for 24 h. Black precipitates were obtained after the hydrothermal treatment, filtered, washed with deionized water, and dried at 80 °C for 12 h. The final pure MoS2 powders were prepared by calcination at 700 °C for 4 h in argon atmosphere.2.2 Preparation of Na3V2O2x(PO4)2F3−2x/C cathode material
To prepare the Na3V2O2x(PO4)2F3−2x/C composite by hydrothermal reaction, the precursor, [V(PO3)3]n/C composite, is firstly prepared by the solid-state reaction with stoichiometric amounts of V2O5, NH4H2PO4, and super P carbon. This mixture was annealed twice under N2 atmosphere at 300 and 850 °C. After that, Na3V2O2x(PO4)2F3−2x/C composite was prepared under hydrothermal conditions by reacting NaF and [V(PO3)3]n/C in a 3.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio. The reaction mixture was sealed in a polytetrafluoroethylene (PTFE)-lined steel pressure vessel, which was maintained at 170 °C for 72 h. This carbon content of precursor material prevents the complete oxidation from V3+ to V4+ in presence of water. Then the product was dried at 80 °C for 12 h in an electric oven.
1 molar ratio. The reaction mixture was sealed in a polytetrafluoroethylene (PTFE)-lined steel pressure vessel, which was maintained at 170 °C for 72 h. This carbon content of precursor material prevents the complete oxidation from V3+ to V4+ in presence of water. Then the product was dried at 80 °C for 12 h in an electric oven.
2.3 Characterization
Structural characterization of the materials was performed by the powder X-ray diffraction (XRD) employing Cu-Kα (1.54 Å) radiation (RIGAKU, D/MAX-2500 powder X-ray diffractometer). Particle size and morphology analysis were characterized employing a field emission scanning electron microscope (FE-SEM Hitachi S-4800, Japan) and a transmission electron microscope with energy dispersive spectroscopy (TEM), JEOL 2010F HRTEM, Japan, with a 300 kV operating voltage. The Raman spectra of powders were recorded at room temperature employing a HR 800 Raman spectrophotometer (Jobin Yvon-Horiba, France) using monochromatic He–Ne LASER (633 nm), operating at 20 mW.2.4 Electrochemical measurements
The electrochemical studies of the synthesized MoS2 flowers were analyzed in CR2032 coin cells. The composite electrode was prepared by mixing 80 wt% of active material with 10 wt% of super P carbon and 10 wt% of PVDF, PEO and sodium alginate binders in suitable solvent. The obtained slurry was coated on a piece of Cu foil and cut into 12 mm diameter circular electrodes. Sodium metal was used as an anode and 1 M NaClO4 in propylene carbonate (PC) with 2 vol% fluoroethylene carbonate (FEC) was used as electrolytes. CR2032 cells were assembled in an argon-filled dry glove box (M.O. Tech, South Korea) using Whatman GF/D borosilicate glass-fibre separator. The cyclic voltammetry and ex situ electrochemical impedance spectroscopy at various potentials during first discharge–charge cycle were measured using Biologic Science Instruments (Model: VMP3) between 1 MHz and 2 mHz under AC stimuli with 5 mV of amplitude. The cells were galvanostatically cycled (WBCS3000, Automatic battery cycler system, Wonatech, South Korea) between 3 V and 0.002 V. The Na3V2O2x(PO4)2F3−2x/C composite was used as a cathode material in testing Na-ion full cells. The detailed procedure to make a full-cell is described in our previous paper.26 The mass loading for the cathode and the anode is 2.2 mg and 2.8 mg, respectively, and the area for the cathode and the anode is 1.13 cm2 and 1.54 cm2, respectively. The MoS2/Na3V2O2x(PO4)2F3−2x full-cell was galvanostatically tested within the voltage of 1.0 and 3.0 V at 25 °C. All gravimetric capacity was calculated by the weight of the cathode.3. Results and discussion
3.1 Structural analysis
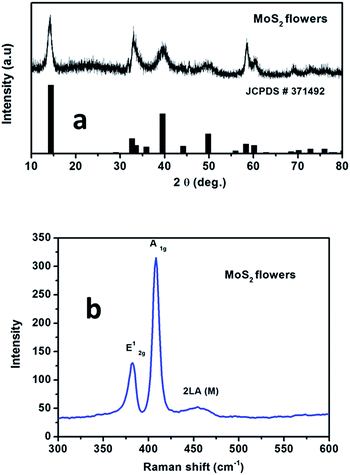
Fig. 1a shows the XRD patterns of the MoS2 microflowers which are indexed with hexagonal structure with the space group P63/mmc (JCPDS #37-1492). Raman spectrum for the pure MoS2 microflowers is shown in Fig. 1b. The peaks at 385.6 cm−1 (E12g) and 408 cm−1 (A1g) are originated from the vibration of Mo–S in-plane and out-of-plane vibration modes respectively. Furthermore, the peak at around 454 cm−1 (2LA) is observed due to the resonance Raman (RR) scattering while using 633 nm laser source.27,28 Hence, it is confirmed that the prepared sample is in pure single phase. The microstructure of as-prepared MoS2 microflowers was obtained from both SEM and TEM micrographs. Fig. 2 shows the SEM images for pure MoS2 microflowers in different magnifications. The size of typical microflowers is approximately 3 μm in width. TEM images of prepared MoS2 microflowers samples were shown in Fig. 3. The HRTEM image of the MoS2 microflowers (Fig. 3c) indicates the formation of flowers with petal morphology of ∼10 nm in thickness. From Fig. 3d, the observed Debye–Scherrer rings represent the (002), (100), (103), (006) and (110) lattice planes and confirm the phase and purity of MoS2 microflowers.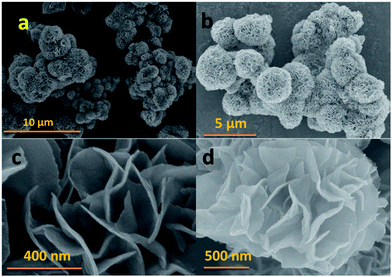 | ||
| Fig. 2 SEM images of prepared MoS2 microflowers with nano-sized petals in different magnifications (a–d). | ||
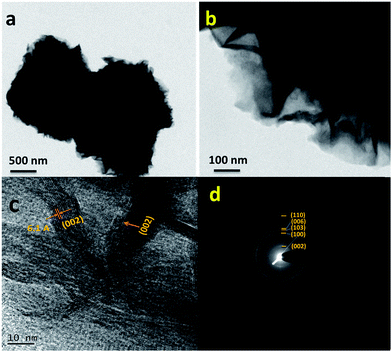 | ||
| Fig. 3 (a and b) TEM images in different magnifications, (c) high resolution image, and (d) SAED patterns for the MoS2 microflowers. | ||
3.2 Electrochemical activities
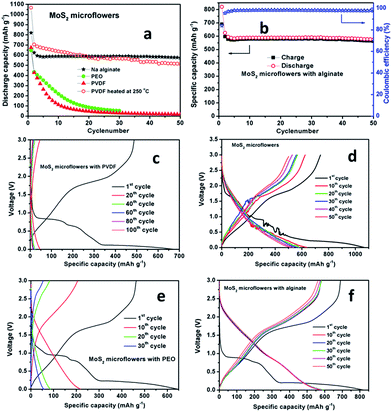
Fig. 4 presents cyclic voltammetry (CV) plots of MoS2 microflowers with PVDF, PEO, heat treated PVDF (i.e. electrode prepared by PVDF and heated at 250 °C for 3 h), and Na-alginate binders, respectively. In the first scan, three cathodic peaks were observed at 0.8, 0.65, and 0.15 V for MoS2 with PVDF and PEO, the first two peaks at 0.8 V and 0.65 V belong with the sodium intercalation into the MoS2 structure and the third peak at 0.15 V corresponds to the conversion reactions of Na+ and Mo4+ to their metallic state along with the formation of Na2S.18 The broad anodic peak at around 1.8 V for two samples (PVDF and PEO) can be ascribed to the oxidation reactions of metallic Mo, which is corresponding to oxidation at 2.2 V in lithium ion batteries.29 From Fig. 4, the cathodic peaks of MoS2 with PVDF and PEO binder are shifting to higher potentials with reducing their intensities from the second scan onwards, which is indicating that lower electrochemical stabilities of these materials. In case of electrodes with both heated PVDF and Na-alginate, they show only one redox peak at 0.35 V and 0.65 V, respectively.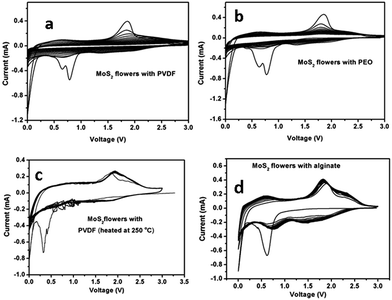 | ||
| Fig. 4 Cyclic voltammetry plots for MoS2 microflowers with (a) PVDF, (b) PEO, (c) PVDF heated electrode at 250 °C and (d) Na-alginate. | ||
In Fig. 4c and d, the broad anodic peaks observed between 1.6 and 2.1 V correspond to the reduction of S to Na2S from the second cycle. Other anodic peaks at 0.8 V and 0.1 V are attributed to the association of Na with Mo. Among the three cathodic peaks, the peaks at 0.5 and 1.65 V represent the oxidation of Mo and the peak at 2.3 V is due to the conversion from element S8 to polysulfide and then to Na2S.30 The peak intensity is stable for the MoS2 microflowers with PVDF heat-treated electrode and Na-alginate binder, still the PVDF heat-treated electrode has small noise due to the formation of very small oxide whiskers on copper foil surface at 250 °C.31 Hence, these cyclic voltammetry results indicate that MoS2 microflowers with alginate binder exhibits better electrochemical properties than other binders due to the high surface interaction between microflowers and Na-alginate binder.32,33
The mechanism of the conversion reaction of MoS2 microflowers with sodium is different with the insertion/deinsertion of sodium into host structures reactions. The reversible electrochemical reaction mechanism of Na with the metal sulfides is as follows.
| MoS2 + 2Na+ + 2e− ↔ Na2MoS2 | (1) |
| Na2MoS2 + 2Na+ + 2e− ↔ Mo + 2Na2S | (2) |
The cycling performances of MoS2 microflowers with different binders are shown in Fig. 5. Fig. 5a exhibits the cyclic stability plots for MoS2 microflowers with PVDF, heated-PVDF, PEO, and Na-alginate binder, respectively. The MoS2 microflowers with Na-alginate and PVDF heat-treated at 250 °C electrode show the higher cyclic stability than the electrodes using conventional PVDF and PEO as binders. Even, Na-alginate binder is showing better stability compared to the PVDF heat-treated electrode after 50 cycles. MoS2 microflowers with all binders were cycled at 0.1C rate (67 mA g−1). By using PVDF as binder, MoS2 microflowers deliver an initial discharge capacity of 680 mA h g−1, but later on, it shows a negligible discharge capacity of 9 mA h g−1 with high capacity fading after 50 cycles. Fig. 5c shows the charge–discharge curves of MoS2 microflowers with PVDF binder up to 50 cycles. MoS2 microflowers with PVDF binder heat-treated at 250 °C for 3 h deliver the first discharge capacity of 1065 mA h g−1 which is higher than its theoretical capacity. At first discharge, MoS2 microflowers might play a role as an electrochemical catalyst for the reversible conversion of some solid electrolyte interface components thereby it could contribute on additional discharge capacity.34 After that, a reversible capacity of 495 mA h g−1 was maintained after 50 cycles with 98% coulombic efficiency (Fig. 5d), revealing its high cycle stability nature as previously reported with Fe3O4 conversion anodes.35 While MoS2 microflowers with PEO binder deliver an initial discharge capacity of 650 mA h g−1, but the discharge capacity is significantly decreased to 44 mA h g−1 after 30 cycles (Fig. 5e). The charge–discharge profile for the MoS2 microflowers with Na-alginate binder for 50 cycles is represented in Fig. 5f. The MoS2 microflowers with Na-alginate binder deliver an initial discharge capacity of 820 mA h g−1 and retain 595 mA h g−1 after 50 cycles with 99% coulombic efficiency, indicating its excellent cycle stability.
The MoS2 microflowers with Na-alginate binder exhibit the considerable performance even at high current rates. The discharge capacities of the MoS2 microflowers with Na-alginate binder at a current of 670 mA g−1 (1C), 1340 mA g−1 (2C), 3350 mA g−1 (5C) and 6700 mA g−1 (10C) for 10 cycles, respectively, are shown in Fig. 6. Without any carbonaceous materials, the MoS2 microflowers with Na alginate binder deliver a stable discharge capacity of 240 mA g−1 at high current rate of 6700 mA g−1 (10C). It is noted that nearly the same capacity can be regained for the electrode when the current density is turned back to 670 mA g−1 and the plateau region in all discharge curves can be observed at high current rates, indicating superior stability of the MoS2 microflowers/alginate anode. Again, the enhancement of electrochemical performances in MoS2 microflowers/alginate anode might be attributed to the strong bonding between active materials and carboxyl groups on binder surface during cycling.32
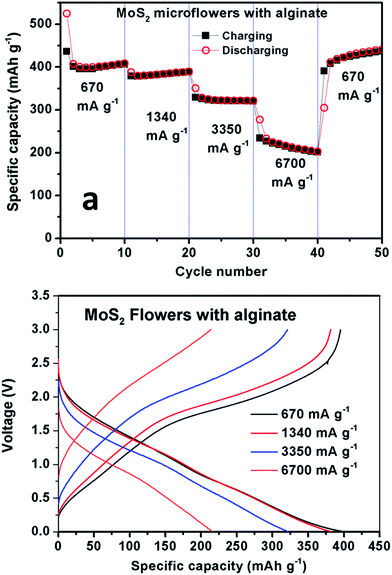 | ||
| Fig. 6 (a) Rate capability and (b) charge–discharge voltage curves at different current rates for the MoS2 microflowers with Na-alginate binder. | ||
In order to understand the electrochemical behavior of the MoS2 microflowers with alginate binder during cycling, electrochemical impedance spectroscopy (EIS) measurements were carried out during first cycle at different voltages, as shown in Fig. 7.
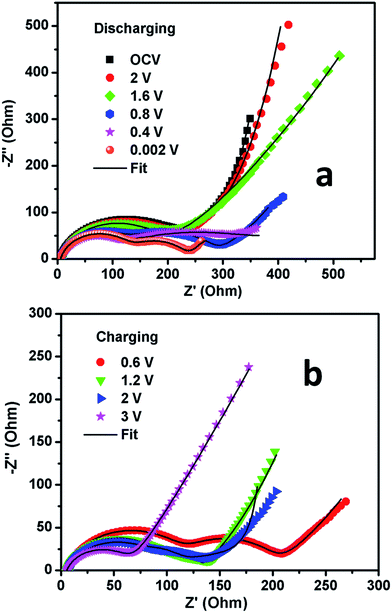 | ||
| Fig. 7 Nyquist plots for the MoS2 microflowers with Na-alginate binder electrode vs. Na/Na+ while (a) discharging and (b) charging states. | ||
The scatters in Fig. 7 represent the experimental data points and the solid lines represent the fitted data in the Nyquist plots for the MoS2 microflowers/alginate. The latter were analyzed by fitting with an electrical equivalent circuit consisting of resistors and constant phase elements displayed in Fig. S1.† The electrical equivalent circuit elements are electrolyte resistance (Re), the separable surface film (SF) and charge transfer (CT) impedances RSF and RCT, also constant phase elements CPESF and CPECT and the finite Warburg impedance (Wd). The depressed semi-circles in the spectra were represented by parallel combination of constant phase elements (CPE's) and resistance. The experimental data was fitted by using Z-fit software with fitting except at low frequencies.
In Fig. 7a, the MoS2 microflowers/alginate impedance results showed that the electrolyte resistance (Re) remained almost constant at 5.4 Ω irrespective of voltage while discharging or charging. Two semi-circles were observed in high and middle frequency range from Fig. 7a. The first semicircle at higher frequency is related to the formation of passivation film on the surface (SF) and the charge transfer process at interface (CT). The second semicircle at low frequency is related to electronic properties of the materials, which is represented as bulk. The Nyquist plot of the cell at OCV consists of a depressed semi-circle followed by Warburg element at low frequencies. This is attributed to the surface film impedance and the values as per fitting are shown in Table S1.† At OCV, only surface film will contribute to the resistance, and it depends on concentration of electrolytes. In addition, solid electrolyte interphase (SEI) formation/partial dissolution/re-formation also takes place on cycling. This is reflected in the changes in the value of surface film impedance (RSF) up on cycling.36,37
Also at 2 V, the impedance plot consists similar to that at OCV. At 1.6 V and 0.8 V, the Nyquist plots contain two semicircles in the high and intermediate frequency range and a sloping line in the low-frequency region. This is exactly matched with the observed CV data which indicate that the earlier stage of conversion reaction and metal nano-particle formation take place at ∼1.2 V. Further discharging to 0.4 and 0.002 V, the size of first semi-circle is remained same, but size of the second semicircle is reduced which might be due to the conversion reaction.
The Nyquist plots of MoS2/alginate anode during first charging state are shown in Fig. 7b. The impedance plots are not following same trend like first discharge, since the mechanism of first-discharge reaction is different from first-charge reaction. At 0.6 V and 1.2 V, the Nyquist plots contain high frequency semicircle along with low frequency depressed semicircle which is due to the formation of the metal oxides from the Mo metal-particles through the conversion reaction. Further charging up to 2.0 V and 3 V, the surface film and charge transfer impedance decreases ascribed to partial dissolution of polymeric gel-type layer. Hence, we concluded that the electrochemical impedance studies can support the observed CV results as well as conversion reaction mechanism.
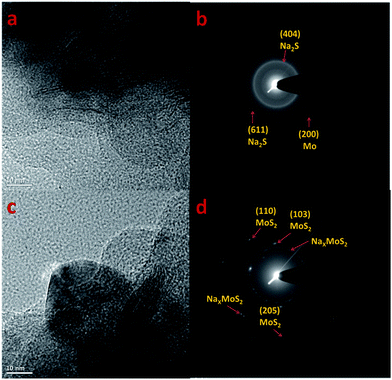
To find the nature of the conversion reaction in the MoS2 microflowers, ex situ TEM analysis were performed on the MoS2 microflowers/alginate electrode at discharging and charging states. The ex situ TEM image and SAED patterns are represented in Fig. 8. The petal morphology is retained after first discharge (Fig. 8a), which indicates the high strong interaction between alginate binder and active materials. The SAED patterns in Fig. 8b shows the inter-planer distance of Mo and Na2S at discharged state. The obtained inter-planer distance values are matched with the Miller indices of pure Mo and Na2S. The ex situ TEM results for the fully charged MoS2 microflowers/alginate electrode are shown in Fig. 8c and d. It is clear that the formation of MoS2 and NaxMoS2 at charged state. Hence, it is proved that the Mo metal and sulfides formed at discharged state and the reformation of MoS2 at charged state.
3.3 Na3V2O2x(PO4)2F3−2x/MoS2 full cell
We have previously studied the feasibility of the Na-ion full cells by combining a negative electrode material, Fe3O4, and a positive electrode material, Na3V2(PO4)3.24 However, the reversible capacity of Fe3O4 was quite low (∼250 mA h g−1), and the battery performance of the Fe3O4/Na3V2(PO4)3 full-cell should be enhanced for cyclic stability. Therefore, we assembled the coin-type full-cell consisting of the MoS2 microflowers anode and the Na3V2O2x(PO4)2F3−2x/C composite cathode. The XRD patterns, SEM image, and electrochemical performances of the Na3V2O2x(PO4)2F3−2x/C composite are summarized in Fig. S2.† Fig. 9a shows the reversible capacity and coulombic efficiency of the MoS2 microflowers/Na3V2O2x(PO4)2F3−2x/C full-cell at a rate of 0.1C (1C corresponds to 130 mA g−1, the theoretical capacity of the Na3V2O2x(PO4)2F3−2x) in a voltage range of 1.0 and 3.0 V. The capacity retention after 40 cycles was ca. 72% (76 mA h g−1), and the average coulombic efficiency was 96%. Fig. 9b shows the voltage profile of the full cell by 40 cycles, indicating an average operating voltage of 1.8 V. The two distinctive voltage plateaus are attributed to the Na3V2O2x(PO4)2F3−2x/C cathode, and the sloppy voltage profiles of MoS2 anode could influence the overall voltage curve of the full-cell.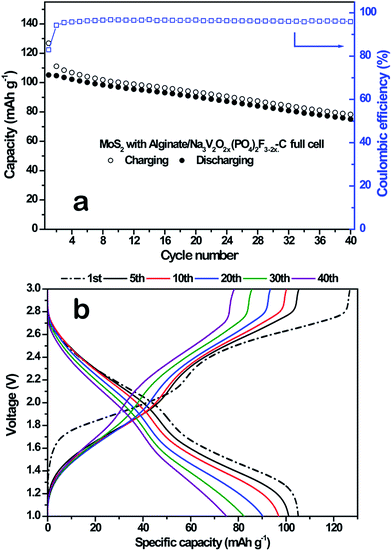 | ||
| Fig. 9 (a) Cyclability and (b) charge–discharge curves for the MoS2 micro flowers with Na-alginate binder/Na3V2O2x(PO4)2F3−2x/C full cell. | ||
4. Conclusions
MoS2 microflowers were prepared by hydrothermal reaction combined with solid state reaction. The prepared MoS2 microflowers were characterized and confirmed the purity of the phase, crystallinity, microstructure and morphology information by XRD, SEM, Raman and HRTEM. Electrochemical properties of MoS2 microflowers with different binders were investigated by using cyclic voltammetry and charge–discharge galvanostatic cycling. While MoS2 microflowers show a high capacity fading with PVDF and PEO binder, it is shown that high capacity was retained for MoS2 with Na-alginate binder. Furthermore, MoS2 microflowers with Na-alginate binder offer good charge transfer kinetics due to the unbreakable electron transport network. MoS2 microflowers/alginate electrodes shows a stable capacity of 595 mA h g−1 at 0.1C (67 mA g−1) rate after 50 cycles. The better performance of MoS2 microflowers/alginate can be ascribed to the stability of the interfaces providing the stable capacity retention in long-term cycling.Acknowledgements
This work was supported by the Program to Solve Climate Changes (NRF-2010-C1AAA001-2010-0029031) of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning. It was also supported by the Climate Change Research Hub of KAIST (Grant No. N01150034).References
- B. L. Ellis and L. F Nazar, Curr. Opin. Solid State Mater. Sci., 2012, 16, 168–177 CrossRef CAS PubMed
.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884–5901 CAS
.
- S. Y. Hong, Y. Kim, Y. Park, A. Choi, N.-S. Choi and K. T. Lee, Energy Environ. Sci., 2013, 6, 2067–2081 CAS
.
- V. L. Chevrier and G. Ceder, J. Electrochem. Soc., 2011, 158, 1011–1014 CrossRef PubMed
.
- L. Xao, Y. Cao and J. Liu, Low-cost Nanomaterials, Green Energy and Technology, Springer-Verlag, London, 2014, pp. 395–424 Search PubMed
.
- C. Masquelier and L. Croguennec, Chem. Rev., 2013, 113, 6552–65915 CrossRef CAS PubMed
.
- V. Palomares, M. Casas-Cabanas, E. Castillo-Martínez, M. H. Han and T. Rojo, Energy Environ. Sci., 2013, 6, 2312–2337 CAS
.
- H. Kim, J. Hong, Y. U. Park, J. Kim, I. Hwang and K. Kang, Adv. Funct. Mater., 2015, 25, 534–541 CrossRef CAS PubMed
.
- P. Senguttuvan, G. Rousse, V. Seznec, J. M. Tarascon and M. R. Palacin, Chem. Mater., 2011, 23, 4109–4111 CrossRef CAS
.
- Q. Sun, Q. Q. Ren, H. Li and Z. W. Fu, Electrochem. Commun., 2011, 13, 1462–1464 CrossRef CAS PubMed
.
- H. Xiong, M. D. Slater, M. Balasubramanian, C. S. Johnson and T. Rajh, J. Phys. Chem. Lett., 2011, 2, 2560–2565 CrossRef CAS
.
- L. Zhao, J. Zhao, Y. S. Hu, H. Li, Z. Zhou, M. Armand and L. Chen, Adv. Energy Mater., 2012, 2, 962–965 CrossRef CAS PubMed
.
- L. Xiao, Y. Cao, J. Xiao, W. Wang, L. Kovarik, Z. Nie and J. Liu, Chem. Commun., 2012, 48, 3321–3323 RSC
.
- B. G. Silbernagel, Solid State Commun., 1975, 17, 361–366 CrossRef CAS
.
- E. Benavente, M. A. Santa Ana, F. Mendizábal and G. González, Coord. Chem. Rev., 2002, 224, 87–109 CrossRef CAS
.
- Y. Miki, D. Nakazato, H. Ikuta, T. Uchida and M. Wakihara, J. Power Sources, 1995, 54, 508–510 CrossRef CAS
.
- U. K. Sen and S. Mitra, ACS Appl. Mater. Interfaces, 2013, 5, 1240–1247 CAS
.
- J. Park, J. S. Kim, J. W. Park, T. H. Nam, K. W. Kim, J. H. Ahn, G. Wang and H. J. Ahn, Electrochim. Acta, 2013, 92, 427–432 CrossRef CAS PubMed
.
- L. David, R. Bhandavat and G. Singh, ACS Nano, 2014, 8, 1759–1770 CrossRef CAS PubMed
.
- J. Wang, C. Luo, T. Gao, A. Langrock, A. C. Mignerey and C. Wang, Small, 2015, 11, 473–481 CrossRef CAS PubMed
.
- X. Xie, Z. Ao, D. Su, J. Zhang and G. Wang, Adv. Funct. Mater., 2015, 25, 1393–1403 CrossRef CAS PubMed
.
- Y. X. Wang, S. L. Chou, D. Wexler, H. K. Liu and S. X. Dou, Chem.–Eur. J., 2014, 20, 9607–9612 CrossRef CAS PubMed
.
- S. H. Choi, Y. N. Ko, J. K. Lee and Y. C. Kang, Adv. Funct. Mater., 2015, 25, 1780–1788 CrossRef CAS PubMed
.
- G. S. Bang, K. W. Nam, J. Y. Kim, J. Shin, J. W. Choi and S. Y. Choi, ACS Appl. Mater. Interfaces, 2014, 6, 7084–7089 CAS
.
- W. H. Pan, M. Leonowicz and E. I. Stiefel, Inorg. Chem., 1983, 22, 612–618 CrossRef
.
- P. R. Kumar, Y. H. Jung, K. K. Bharathi, C. H. Lim and D. K. Kim, Electrochim. Acta, 2014, 146, 503–510 CrossRef CAS PubMed
.
- H. Li, Q. Zhang, C. C. R. Yap, B. K. Tay, T. H. T. Edwin, A. Olivier and D. Baillargeat, Adv. Funct. Mater., 2012, 22, 1385–1390 CrossRef CAS PubMed
.
- K. K. Liu, W. Zhang, Y. H. Lee, Y. C. Lin, M. T. Chang, C. Y. Su, C. S. Chang, H. Li, Y. Shi, H. Zhang, C. S. Lai and L. J. Li, Nano Lett., 2012, 12, 1538–1544 CrossRef CAS PubMed
.
- Y. X. Wang, K. H. Seng, S. L. Chou, J. Z. Wang, Z. Guo, D. Wexler, H. K. Liu and S. X. Doua, Chem. Commun., 2014, 50, 10730–10733 RSC
.
- J. Xiao, X. Wang, X. Q. Yang, S. Xun, G. Liu, P. K. Koech, J. Liu and J. P. Lemmon, Adv. Funct. Mater., 2011, 21, 2840–2846 CrossRef CAS PubMed
.
- E. A. Gulbransen, T. P. Copan and K. F. Andrew, J. Electrochem. Soc., 1961, 2, 119–123 CrossRef PubMed
.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed
.
- P. Ramesh Kumar and S. Mitra, RSC Adv., 2013, 3, 25058–25064 RSC
.
- L. Su, Y. Zhong and Z. Zhou, J. Mater. Chem. A, 2013, 1, 15158–15166 CAS
.
- S. Hariharan, K. Saravanan, V. Ramar and P. Balaya, Phys. Chem. Chem. Phys., 2013, 15, 2945–2953 RSC
.
- S. Leroy, F. Blanchard, R. Dedryvère, H. Martinez, B. Carré, D. Lemordant and D. Gonbeau, Surf. Interface Anal., 2005, 37, 773–781 CrossRef CAS PubMed
.
- D. Aurbach, A. Nimberger, B. Markovsky, E. Levi, E. Sominski and A. Gedanken, Chem. Mater., 2002, 14, 4155–4163 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: An equivalent circuit, XRD, SEM and CV of Na3V2O2x(PO4)2F3−2x sample. See DOI: 10.1039/c5ra16297a |
| This journal is © The Royal Society of Chemistry 2015 |