Transforming linoleic acid into a nanoemulsion for enhanced activity against methicillin susceptible and resistant Staphylococcus aureus
Sandeep J. Sonawane,
Rahul S. Kalhapure,
Mahantesh Jadhav,
Sanjeev Rambharose,
Chunderika Mocktar and
Thirumala Govender*
Discipline of Pharmaceutical Sciences, School of Health Sciences, University of KwaZulu-Natal, Private Bag X54001, Durban 4000, KwaZulu-Natal, South Africa. E-mail: govenderth@ukzn.ac.za; Fax: +27 31 260 7792; Tel: +27 31 260 7357 Tel: +27 31 260 7358
First published on 19th October 2015
Abstract
The activity of antibacterial agents can be enhanced by transforming them into the nano form. The aim of this study was therefore to enhance the antibacterial activity of linoleic acid (LA) against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) by formulating it as a nanoemulsion (NE). The mean globule diameter, polydispersity index and zeta potential of the optimized LA NE containing benzalkonium chloride (BAC) as a stabilizer were 75.14 ± 3 nm, 0.145 ± 0.01 and 45.7 ± 1.27 mV respectively. The turbidity absorbance, conductivity and viscosity were 1.773 ± 0.69, 0.0508 ± 0.006 mS cm−1 and 92.74 ± 2.17 mPas respectively, and the formulation was stable at 4 °C for 3 months. The LA NE was non-toxic and exhibited a 205-fold greater increase in the antibacterial activity than plain LA against S. aureus and MRSA. The fractional inhibitory concentration values indicated that the combination of LA and BAC had a synergistic effect. The molecular modeling studies revealed better stability of the LA–BAC system than LA with other surfactants. Bacterial protein degradation and cell morphology studies confirmed that the antibacterial activity of LA NE was due to cell membrane damage. These findings suggest that the developed LA NE could be a promising non-antibiotic drug containing antibacterial nano delivery system.
1. Introduction
Infectious diseases continue to be one of the major health challenges in both developed and developing countries.1 The industrial production of penicillin, and subsequent development of stronger antibiotics, led to significant success in controlling infectious diseases until the 1970–1980s.2 However, increasing use and/or abuse of antibiotics has resulted in the development of resistant bacterial strains, such as methicillin-resistant Staphylococcus aureus (MRSA),3 vancomycin-resistant Enterococcus (VRE)4 and vancomycin-resistant Staphylococcus aureus (VRSA).5Another problem in antimicrobial therapy is that frequent high doses of intravenous antibiotics may produce toxic plasma drug concentrations, making complete suppression of the infection under such conditions difficult to achieve due to the bacteria's ability to form biofilms.6 Novel approaches for treating microbial infections are therefore necessary, due to the increasing spread of resistance to currently used antibiotics, the slow development rate of newer antibacterials, and the possibility of resistance to future new antimicrobial drugs.7
Nanotechnology, the science dealing with the design, production and application of materials in the nano-scale,8 is an attractive strategy to effectively control and optimize infectious diseases treatment.2 The physicochemical properties that make nano sized drug delivery systems an effective tool to overcome bacterial infections and challenges associated with them are: (a) small size and high surface area-to-mass ratio;8 (b) significant interaction with microorganisms; and (c) possibility of structural and functional system modification.9 Apart from metal nanoparticles (NPs), different antibiotic loaded nanosystems, such as nanoemulsions (NEs), solid lipid nanoparticles (SLNs), liposomes, polymer nanoparticles (PNPs), dendrimers, lipid polymer hybrid nanoparticles (LPHNs), micellar systems, nanostructures made up of pure carbon [carbon nanotubes (CNTs), nanosheets, and nanorods] and nanohybrids, have been reported to address the problem of bacterial infections. The details of these nanoantibiotics can be found elsewhere in the literature.2,9–11
NEs, which are heterogeneous metastable submicron oil-in-water dispersions with globule diameter in the range of 10–100 nm,12 are increasingly being used to treat bacterial infections13–15 due to their important properties namely: biodegradability, biocompatibility, ease of preparation and physical stability.10 The NEs long-term physical stability, high bioavailability and low turbidity are properties that make them superior to conventional emulsions, and therefore extensively used as attractive systems in the food, cosmetics and pharmaceutical industries.15 The antimicrobial NEs are an oil-in water type, with nano-sized positively charged globules having a broad spectrum activity against enveloped virus, fungi and bacteria.16 High energy methods, such as ultrasonication, shearing and homogenization, can be employed to prepare antibacterial NEs.14
Surfactants form an integral part of NEs by acting as stabilizers for the formed globules, a critical factor being their concentration, with higher levels possibly causing systemic and topical toxicity. Large quantities of surfactants in NEs may result in irritation of the gastrointestinal tract and skin when administered orally and topically respectively.17 It is therefore important to optimize the surfactant level in NEs and to use the minimum concentration. Another important criterion for NE formulation is selecting a surfactant with the correct hydrophilic–lipophilic balance (HLB) value to improve its stability, this being greater than 10.18 Two types of surfactants viz., non-ionic and ionic (cationic and anionic), have been reported to formulate essential oil NE,13,19 with the former being comparatively less toxic than the latter.17 The toxicity order of surfactants is cationic > anionic > nonionic,20 and although more toxic than nonionic, various ionic surfactants, such as sodium lauryl sulfate (anionic) and benzalkonium chloride (cationic), have been reported as antibacterials themselves.19,21 Although ionic surfactants are comparatively more toxic than nonionic surfactants, they may influence the antibacterial activity of essential oil NE by changing the electrical characteristics of the oil droplets.19 Cationic surfactants may increase the antibacterial activity of NE by electrostatically attracting themselves to the surface of negatively charged bacterial cell walls.19 The application of cationic surfactants in formulating essential oil or fatty acid NE could therefore be an attractive strategy to enhance the antibacterial activity, either by altering the electrical characteristics of the oil droplets or by a synergistic effect with an antibacterial fatty acid.
Linoleic acid (LA), an essential diunsaturated fatty acid, is the major component of many common seed oils.22 It has been reported to be an effective antibacterial unsaturated fatty acid (FA) against Staphylococcus aureus and MRSA in combination with vancomycin (VCM)7 as well as alone.23 The FAs have been reported to act by various mechanisms, including disrupting the electron transport chain and oxidative phosphorylation,24 decreasing the transfer frequency of conjugal DNA25 and inhibiting bacterial enoyl-acyl carrier protein reductase (FabI).23 It has been reported that the antibacterial activity of antibacterial agents can be enhanced by transforming them into the nano form.26 While the antibacterial activity applications of LA have been reported either alone as an antibacterial agent23 or as an adjuvant in drug loaded nanosystems,7 its transformation into a nanoemulsion has not been reported. Following an extensive literature search, it appears that only the liposomal formulation of LA alone for enhanced skin whitening effect has been reported,27 and that the antibacterial activity of LA alone in its nanoform remains to be exploited.
NEs as a drug carrier have a number of advantages over liposomal formulations, including ease of manufacture, formulation stability, high loading capacity, and relatively low complexity.28,29 The purpose of the present investigation was therefore to prepare LA as a NE, and to characterize it in terms of its physical and antibacterial properties.
2. Experimental
2.1 Materials
LA, Lutrol F68 and benzalkonium chloride (BAC) were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, USA), Tween 80 was purchased from Merck (Hohenbrunn, Germany) and sodium lauryl sulfate (SLS) from Saarchem (Pty) Ltd. (Krugersdorp, South Africa). Mueller–Hinton Agar (MHA) and Nutrient Broth were obtained from Biolab Inc., (Modderfontein, South Africa), and Mueller–Hinton broth (MHB) from Oxoid Ltd., (Basingstoke, England). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Merck Chemicals (Darmstadt, Germany), and all other reagents and chemicals were from Sigma-Aldrich Co. Ltd. (St. Louis, USA). The water was obtained from an Elix® water purification system by Millipore Corp., (Massachusetts, USA), and the bacterial cultures S. aureus (ATCC 25922) and S. aureus Rosenbach (ATCC®BAA-1683TM) (MRSA) were used in antibacterial studies.2.2 Methods
2.2.1.1 Screening of surfactants. Four surfactants, namely: [Tween 80 (nonionic), Lutrol-F68 (nonionic), SLS (anionic) and BAC (cationic)], were screened for preparing LA NE and assessing their antibacterial potential against S. aureus and MRSA. The LA concentration (5% w/w) was kept constant for all the formulations. LA was mixed with each surfactant in different ratios (w/w) 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25, 1
0.25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75, 1
0.75, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, followed by adding water to obtain a coarse emulsion. This was further sonicated using a probeOmni Sonic Ruptor 400 Ultrasonic Homogenizer (Kennesaw, USA) for 20 min (30% amplitude) at 20 °C. The temperature of the emulsification process was monitored by using a thermometer, and an ice bath was used to control the heat generated in this high energy process.
1, followed by adding water to obtain a coarse emulsion. This was further sonicated using a probeOmni Sonic Ruptor 400 Ultrasonic Homogenizer (Kennesaw, USA) for 20 min (30% amplitude) at 20 °C. The temperature of the emulsification process was monitored by using a thermometer, and an ice bath was used to control the heat generated in this high energy process.
2.2.1.2 Determination of mean globule diameter, polydispersity index and zeta potential. The mean globule diameter (MGD), polydispersity index (PI) and zeta potential (ZP) of all the NEs were determined by dynamic light scattering experiments. NEs were suitably diluted with water and all measurements were performed in triplicate at 25 °C using a Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcestershire, UK) equipped with a 633 nm laser and 173° detection optics. PI and ZP measured the size distribution and overall charge acquired by the nanoparticles respectively.
2.2.1.3 Effect of surfactants on antibacterial activity of LA. In order to assess the effect of the surfactants on the antibacterial activity of LA, the minimum inhibitory concentration (MIC) values for all NEs were determined against S. aureus and MRSA as described previously.7 In brief, bacterial cultures grown overnight in nutrient broth at 37 °C were adjusted to 0.5 McFarland. The prepared serial dilutions of the test samples were incubated with bacterial cultures at 37 °C for 18 h in a shaking incubator at 100 rpm. The dilutions (10 μl) were spotted on MHA plates and incubated for 18 h to determine the MIC. Bare LA and surfactant solutions were used as controls, and all the experiments were repeated thrice.
2.2.2.1 Turbidity. Turbidity analysis of the optimized LA NE was performed by measuring the absorbance of undiluted samples at 600 nm using a UV-visible spectrophotometer (Schimadzu UV 1601, Tokyo, Japan). Each measurement was performed in triplicate and the results were calculated as the mean ± SD.
2.2.2.2 Conductivity. The conductivity of the optimized LA NE formulation was determined at 25 °C using a Zetasizer Nano ZS90 (Malvern Instruments Ltd., Worcestershire, UK) equipped with a 633 nm laser and 173° detection optics. NEs were suitably diluted with water, and all measurements were performed in triplicate and are represented as mean ± SD.
2.2.2.3 Rheology. Viscosity measurement of the optimized LA NE was performed with a MCR 302 Rheometer® (Anton Parr, Graz, Austria) using the parallel plate (PP50) configuration by applying the shear rate in a linear manner from 0 to 100 s−1 at 25 °C. The apparent viscosity of the NE was calculated at shear rate 100 s−1. All the experiments were performed in triplicate and are represented as mean ± SD.
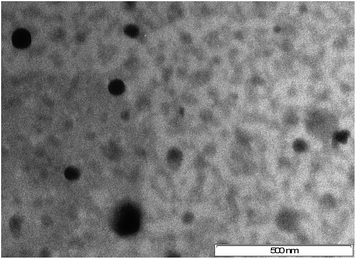
2.2.2.4 Morphology. Surface morphology of LA NE was examined at 25 ± 2 °C using a mega view III camera of transmission electron microscope (TEM) (Jeol, JEM-1010, Tokyo, Japan). To obtain TEM images, a drop of appropriately diluted NE was placed on 3 mM forman (0.5% plastic powder in amyl acetate) coated copper grid (300 mesh), allowed to dry, then stained with 1% w/v phosphotungstic acid and visualized at pH 3.2 with magnification of 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× using an accelerating voltage of 100 kV.
000× using an accelerating voltage of 100 kV.
2.2.2.5 Stability.
2.2.2.5.1 Accelerated conditions. The optimized LA NE formulations prepared by ultrasonic emulsification were centrifuged (10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 25 ± 2 °C for 30 min, and the resistance of emulsion to centrifugation was studied by measuring its MGD, PI and ZP.
000 rpm) at 25 ± 2 °C for 30 min, and the resistance of emulsion to centrifugation was studied by measuring its MGD, PI and ZP.
2.2.2.5.2 Physical stability. The stability of the optimized LA NE was evaluated at room temperature (RT) and at 4 °C over a period of 3 months, while physical appearance, MGD, PI and ZP were used as assessment parameters for stability.
2.2.2.6 Microbiological evaluation.
2.2.2.6.1 MIC determination. The MIC values for optimized LA NEs were determined against S. aureus and MRSA by broth dilution method as described under Section 2.2.1.3.
2.2.2.6.2 Fractional inhibitory concentration index (ΣFIC). The effect of the combination of LA and BAC was studied by determining the ΣFIC30 using the following equations and Table 1.31
| FIC(LA) = MIC of LA in combination with BAC/MIC of LA alone | (1) |
| FIC(BAC) = MIC(BAC) in combination with LA/MIC of BAC alone | (2) |
| ΣFIC = FIC(LA) + FIC(BAC) | (3) |
| Index | Synergy | Additive | Indifference | Antagonism |
|---|---|---|---|---|
| FIC | ≤0.5 | >0.5–1 | >1 to < 2 | ≥2 |
2.2.2.6.3 Bacterial protein degradation. To determine the cell membrane damage to S. aureus and MRSA, SDS-PAGE study of the bacterial proteins was performed after incubating the bacterial cells with the LA NE (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), according to a previously reported procedure.32 The cultures of the S. aureus and MRSA strains were grown for overnight incubation at 37 °C. An aliquot (0.1 ml) of the overnight grown (1 × 109 cfu ml−1) bacterial suspension (S. aureus and MRSA) was inoculated into 10 ml of fresh MHB and incubated at 37 °C for 24 h. The bacterial cells separated by centrifugation (8000 rpm) at 25 ± 2 °C for 2 min were re-suspended into 10 ml of sterile saline solution (8.5 g NaCl l−1). To this sterile suspension of S. aureus and MRSA, 3.05 μg ml−1 and 1.52 μg ml−1 (quantities equivalent to MIC against respective bacteria) of LA NE (1
1), according to a previously reported procedure.32 The cultures of the S. aureus and MRSA strains were grown for overnight incubation at 37 °C. An aliquot (0.1 ml) of the overnight grown (1 × 109 cfu ml−1) bacterial suspension (S. aureus and MRSA) was inoculated into 10 ml of fresh MHB and incubated at 37 °C for 24 h. The bacterial cells separated by centrifugation (8000 rpm) at 25 ± 2 °C for 2 min were re-suspended into 10 ml of sterile saline solution (8.5 g NaCl l−1). To this sterile suspension of S. aureus and MRSA, 3.05 μg ml−1 and 1.52 μg ml−1 (quantities equivalent to MIC against respective bacteria) of LA NE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added respectively. The untreated suspension of S. aureus and MRSA were used as controls and incubated at 37 °C for 24 h. An aliquot of 50 μl of the bacterial suspension was heated at 100 °C for 10 min after being combined with 25 μl of the sample buffer pH-6.8 (1 M Tris–HCl, 50% glycerol, 10% SDS, 10% β-mercaptoethanol, 0.1% bromophenol blue). The treated aliquot was loaded in 3% and 12% SDS-PAGE for the preparation of stacking and resolving gel respectively. After running the plates at 10 mA and 20 mA on the stacking gel and resolving gel respectively, protein bands were visualized on the gels by Coomassie brilliant blue R250.
1) were added respectively. The untreated suspension of S. aureus and MRSA were used as controls and incubated at 37 °C for 24 h. An aliquot of 50 μl of the bacterial suspension was heated at 100 °C for 10 min after being combined with 25 μl of the sample buffer pH-6.8 (1 M Tris–HCl, 50% glycerol, 10% SDS, 10% β-mercaptoethanol, 0.1% bromophenol blue). The treated aliquot was loaded in 3% and 12% SDS-PAGE for the preparation of stacking and resolving gel respectively. After running the plates at 10 mA and 20 mA on the stacking gel and resolving gel respectively, protein bands were visualized on the gels by Coomassie brilliant blue R250.
2.2.2.6.4 Bacterial cell morphology. To identify the possible mode of action of the LA NE (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) against S. aureus and MRSA, an analysis of the morphological changes in the bacterial cells after treatment with the LA NE was undertaken according to a literature reported method.33,34 In brief, bacterial cell suspensions were prepared as described under Section 2.2.2.6.3, while the pellets were prepared from untreated and LA NE (1
1) against S. aureus and MRSA, an analysis of the morphological changes in the bacterial cells after treatment with the LA NE was undertaken according to a literature reported method.33,34 In brief, bacterial cell suspensions were prepared as described under Section 2.2.2.6.3, while the pellets were prepared from untreated and LA NE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) treated cell suspensions by centrifugation (8000 rpm) at 25 ± 2 °C for 2 min. The supernatant solution was immediately replaced by 2.5% (w/v) glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.2) to re-suspend the formed pellets and pre-fix the bacterial cells. The samples were again centrifuged (13
1) treated cell suspensions by centrifugation (8000 rpm) at 25 ± 2 °C for 2 min. The supernatant solution was immediately replaced by 2.5% (w/v) glutaraldehyde solution in 0.1 M phosphate buffer (pH 7.2) to re-suspend the formed pellets and pre-fix the bacterial cells. The samples were again centrifuged (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 25 ± 2 °C for 30 min to obtain the pellets, and allowed to stand overnight at 4 °C. The following day, the pellets were washed thrice with 0.1 M phosphate buffer solution (pH 7.2), after which they were embedded in 2% agarose, cut into small pieces and post fixed for 1 h in 4% osmium tetroxide (OsO4) in 0.1 M phosphate buffer solution (pH 7.2). After 1 h, the samples were washed thrice with 0.1 M phosphate buffer solution (pH 7.2). The dehydration of the cells was performed using increasing concentrations of aqueous acetone solutions (30, 50, 75, and 100%). These dehydrated samples were embedded in Spurr's resin, cut into thin sections using a diamond knife, and placed on a 3 mM forman (0.5% plastic powder in amyl acetate) coated copper grid (300 mesh). Finally, the samples were stained with uranyl acetate and analyzed using a TEM (Jeol, JEM-1010, Tokyo, Japan) at an accelerating voltage of 100 kV.
000 rpm) at 25 ± 2 °C for 30 min to obtain the pellets, and allowed to stand overnight at 4 °C. The following day, the pellets were washed thrice with 0.1 M phosphate buffer solution (pH 7.2), after which they were embedded in 2% agarose, cut into small pieces and post fixed for 1 h in 4% osmium tetroxide (OsO4) in 0.1 M phosphate buffer solution (pH 7.2). After 1 h, the samples were washed thrice with 0.1 M phosphate buffer solution (pH 7.2). The dehydration of the cells was performed using increasing concentrations of aqueous acetone solutions (30, 50, 75, and 100%). These dehydrated samples were embedded in Spurr's resin, cut into thin sections using a diamond knife, and placed on a 3 mM forman (0.5% plastic powder in amyl acetate) coated copper grid (300 mesh). Finally, the samples were stained with uranyl acetate and analyzed using a TEM (Jeol, JEM-1010, Tokyo, Japan) at an accelerating voltage of 100 kV.
2.2.2.7 Molecular modeling. The molecular modeling was performed to support the LA–surfactant complex formation and to identify the stable LA–surfactant system in the NE formulations. The ChemDraw (ChemBio 3D Ultra14) and Avogadro (v.1.0.3) molecular mechanics programs were used to draw the structures (LA, BAC, SLS and Tween 80) and calculate the interaction energy respectively. MMF94s force field35 was used for geometry-optimization and energy calculations. The potential energies of individual molecules are associated with their position, composition and atom arrangement.36 The potential energies of all the individual surfactants alone and in combination with LA were calculated by using their geometrically optimized structures. Furthermore, the interaction energies (total potential energy deviation), Eint, were calculated mathematically as the difference between the total potential energy of the LA complex with surfactant (ELAS) and the sum of the potential energies of isolated individual molecule (ELA + ES)37 using the equation:
| Eint = ELAS − (ELA + ES) | (4) |
The molecular stability was determined by comparing the total potential energies of the isolated and complexed systems. The complex formation and its stability were favored with a reduction in the complex interaction energy (Eint).38
2.2.2.8 In vitro cytotoxicity. In vitro cytotoxicity of the LA NE (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) against human liver hepatocellular carcinoma (HepG2), human breast adenocarcinoma (MCF 7) and human cervix adenocarcinoma (HeLa) cell lines was determined using an MTT assay. All three cell lines were harvested from the exponential growth phase and seeded equivalently (2.5 × 103) into a 96-well plate and incubated for 24 h to allow for adherence. Thereafter, the culture medium was removed and replaced with the fresh medium (100 μl per well), and the LA NE was added to the wells to achieve final concentrations ranging from 20 to 100 μg ml−1. The control wells were prepared by adding the culture medium only, and wells containing the culture medium without cells were used as blanks. The plate was further incubated for 48 h, after which the culture medium and compounds were removed, replaced with the fresh medium (100 μl) and 5 mg ml−1 MTT solution in PBS (100 μl). The MTT solution was removed, and DMSO (100 μl) was added to each well to solubilize the MTT formazan after 4 h incubation. The optical density of each well was measured on a microplate spectrophotometer (Mindray MR-96A) at a wavelength of 540 nm.39 The percentage cell viability was calculated as follows:
1) against human liver hepatocellular carcinoma (HepG2), human breast adenocarcinoma (MCF 7) and human cervix adenocarcinoma (HeLa) cell lines was determined using an MTT assay. All three cell lines were harvested from the exponential growth phase and seeded equivalently (2.5 × 103) into a 96-well plate and incubated for 24 h to allow for adherence. Thereafter, the culture medium was removed and replaced with the fresh medium (100 μl per well), and the LA NE was added to the wells to achieve final concentrations ranging from 20 to 100 μg ml−1. The control wells were prepared by adding the culture medium only, and wells containing the culture medium without cells were used as blanks. The plate was further incubated for 48 h, after which the culture medium and compounds were removed, replaced with the fresh medium (100 μl) and 5 mg ml−1 MTT solution in PBS (100 μl). The MTT solution was removed, and DMSO (100 μl) was added to each well to solubilize the MTT formazan after 4 h incubation. The optical density of each well was measured on a microplate spectrophotometer (Mindray MR-96A) at a wavelength of 540 nm.39 The percentage cell viability was calculated as follows:| % cell survival = [A540 nm treated cells]/[A540 nm untreated cells] × 100 | (5) |
2.2.2.9 Statistical analysis. The data is expressed as mean ± standard deviation (SD), and was statistically analyzed using one-way analysis of variants (ANOVA) and non-parametric Mann–Whitney test with GraphPad Prism® (Graph Pad Software Inc. Version 5, San Diego, CA). A p value of less than 0.05 was considered statistically significant.
3. Results and discussion
3.1 Formulation development of LA NE
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 (% w/w) ratio of LA to surfactant showed the highest MGD (Table 2) and NEs with 1
0.25 (% w/w) ratio of LA to surfactant showed the highest MGD (Table 2) and NEs with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA to surfactant exhibited the lowest MGD (Table 2). The results obtained in this study were consistent with previous findings, where the MGD decreased with an increase in surfactant concentration.15,40 ZP measurement of the LA NEs prepared by various surfactants at different concentrations exhibited an increase in ZP with an rise in surfactant concentration (Table 2). Surfactants play an important role in solubilization or stabilization of drugs in colloidal drug delivery system, such as NEs.41 It has been reported that a higher value of ZP reduces the chances of coalescence, and thereby helps to keep the NEs stable by maintaining uniformity of globule size.42 The LA NEs prepared using nonionic (Tween80 and Lutrol F68) and anionic surfactants (SLS) showed negative values of ZP, whereas the LA NE prepared using cationic surfactant (BAC) showed positive values of ZP. These negative and positive zeta potential values are the result of adsorbing ionic surfactants onto the surfaces of the droplets.19
1 (w/w) ratio of LA to surfactant exhibited the lowest MGD (Table 2). The results obtained in this study were consistent with previous findings, where the MGD decreased with an increase in surfactant concentration.15,40 ZP measurement of the LA NEs prepared by various surfactants at different concentrations exhibited an increase in ZP with an rise in surfactant concentration (Table 2). Surfactants play an important role in solubilization or stabilization of drugs in colloidal drug delivery system, such as NEs.41 It has been reported that a higher value of ZP reduces the chances of coalescence, and thereby helps to keep the NEs stable by maintaining uniformity of globule size.42 The LA NEs prepared using nonionic (Tween80 and Lutrol F68) and anionic surfactants (SLS) showed negative values of ZP, whereas the LA NE prepared using cationic surfactant (BAC) showed positive values of ZP. These negative and positive zeta potential values are the result of adsorbing ionic surfactants onto the surfaces of the droplets.19
| Surfactant | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) surfactant (w/w) surfactant (w/w) |
MGD (nm) | PI | ZP (mV) |
|---|---|---|---|---|
| Tween 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
168.2 ± 8.80 | 0.240 ± 0.075 | −35.5 ± 3.08 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
112.7 ± 1.70 | 0.161 ± 0.012 | −28.5 ± 3.32 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
81.95 ± 0.50 | 0.189 ± 0.018 | −29.3 ± 2.10 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
65.07 ± 0.27 | 0.199 ± 0.009 | −23.3 ± 3.36 | |
| Lutrol F68 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
171.0 ± 1.07 | 0.144 ± 0.009 | −31.8 ± 0.51 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
163.8 ± 1.71 | 0.144 ± 0.005 | −28.9 ± 1.08 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
148.5 ± 2.27 | 0.146 ± 0.020 | −27.0 ± 1.01 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
140.3 ± 3.31 | 0.098 ± 0.028 | −24.8 ± 1.00 | |
| SLS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
250.1 ± 1.88 | 0.308 ± 0.032 | −55.9 ± 2.15 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
192.6 ± 0.89 | 0.138 ± 0.012 | −54.7 ± 0.70 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
181.5 ± 2.29 | 0.103 ± 0.015 | −48.3 ± 1.97 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
152.0 ± 1.17 | 0.079 ± 0.013 | −46.8 ± 0.75 | |
| BAC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
151.3 ± 1.15 | 0.097 ± 0.010 | 28.2 ± 1.50 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
136.8 ± 1.15 | 0.197 ± 0.035 | 36.5 ± 3.79 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
94.27 ± 3.68 | 0.156 ± 0.028 | 39.9 ± 5.20 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75.14 ± 3.02 | 0.145 ± 0.011 | 45.7 ± 1.27 |
| Surfactant | LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) surfactant (w/w) surfactant (w/w) |
MICb (μg ml−1) | |
|---|---|---|---|
| Microbial strain | |||
| S. aureus | MRSA | ||
| a NA = no activity.b p < 0.05 when compared to plain LA. | |||
| Tween 80 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
625 | 312.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
3125 | 6250 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
3125 | 6250 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
1562.5 | 6250 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1562.5 | 1562.5 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | NA | |
| Lutrol F68 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
6250 | 1562.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
3125 | 781.25 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
3125 | 3125 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1562.5 | 3125 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
NA | NA | |
| SLS | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
3125 | 3125 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
781.25 | 3125 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
1562.5 | 3125 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1562.5 | 781.25 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3125 | 6250 | |
| BAC | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
781.25 | 195.31 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
48.82 | 24.41 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
24.41 | 3.05 | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3.05 | 1.52 | |
0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6.10 | 6.10 | |
While it was expected that the antibacterial surfactants SLS and BAC could result in enhancing antibacterial activity by synergistic action, in this study, this only occurred in BAC. This may be due to the adsorption of the antimicrobial surfactants onto the surfaces of oil droplets in NE decreasing the amount of surfactant present to interact with the bacteria, thereby affecting their efficacy. The cell wall of S. aureus is negatively charged, and the NE, prepared by using BAC containing positively charged droplets, might therefore be expected to be electrostatically attracted to their surfaces, whereas the NE prepared by using SLS containing negatively charged droplets would be expected to be repelled.19 Although Tween 80 alone did not show any antibacterial activity, when combined with the LA NEs containing different concentrations, it exhibited antibacterial activity. The highest antibacterial activity (lowest MIC) was observed for NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (% w/w) ratio of LA and Tween 80 (MIC value of 1562.5 μg ml−1 against both S. aureus and MRSA) in this series. These results are therefore indicative of the fact that Tween 80 leads to a decrease in antibacterial activity (increased MIC value) of LA. This is similar to previous findings, where loss of effectiveness of triclosan against MRSA was observed when Tween 80 was added.43 LA acts against Gram-positive bacteria by inhibiting FabI,23 and its loss of effectiveness against both S. aureus and MRSA in NE could be attributed to Tween 80 interfering with the mechanism of antibacterial action.43
1 (% w/w) ratio of LA and Tween 80 (MIC value of 1562.5 μg ml−1 against both S. aureus and MRSA) in this series. These results are therefore indicative of the fact that Tween 80 leads to a decrease in antibacterial activity (increased MIC value) of LA. This is similar to previous findings, where loss of effectiveness of triclosan against MRSA was observed when Tween 80 was added.43 LA acts against Gram-positive bacteria by inhibiting FabI,23 and its loss of effectiveness against both S. aureus and MRSA in NE could be attributed to Tween 80 interfering with the mechanism of antibacterial action.43
Similarly, LA NEs prepared using Lutrol F68 and SLS also affected the antibacterial activity of LA (Table 3). The NE, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (% w/w) ratio of LA and Lutrol F68, was the most effective formulation in the series against S. aureus, exhibiting a MIC of 1562.5 μg ml−1, while an NE with a 1
1 (% w/w) ratio of LA and Lutrol F68, was the most effective formulation in the series against S. aureus, exhibiting a MIC of 1562.5 μg ml−1, while an NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (% w/w) ratio of LA and Lutrol F68 was the most active formulation against MRSA, with a MIC value of 781.25 μg ml−1 (Table 3), and bare Lutrol F68 exhibited no activity against both S. aureus and MRSA. The NE formulation containing SLS as a surfactant was expected to be the most effective as SLS on its own has antibacterial activity against S. aureus,13 and it may be possible to obtain a synergistic effect with LA. In our study, MIC values for SLS were found to be 3125 and 6250 μg ml−1 against S. aureus and MRSA respectively, while in the literature against S. aureus it is 156.25 μg ml−1.13 In the SLS series, the NE with a 1
0.5 (% w/w) ratio of LA and Lutrol F68 was the most active formulation against MRSA, with a MIC value of 781.25 μg ml−1 (Table 3), and bare Lutrol F68 exhibited no activity against both S. aureus and MRSA. The NE formulation containing SLS as a surfactant was expected to be the most effective as SLS on its own has antibacterial activity against S. aureus,13 and it may be possible to obtain a synergistic effect with LA. In our study, MIC values for SLS were found to be 3125 and 6250 μg ml−1 against S. aureus and MRSA respectively, while in the literature against S. aureus it is 156.25 μg ml−1.13 In the SLS series, the NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 (w/w) ratio of LA and SLS showed the lowest MIC value against S. aureus (781.25 μg ml−1), and the NE with a 1
0.5 (w/w) ratio of LA and SLS showed the lowest MIC value against S. aureus (781.25 μg ml−1), and the NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA and SLS showed the lowest MIC value against MRSA (781.25 μg ml−1) (Table 3).
1 (w/w) ratio of LA and SLS showed the lowest MIC value against MRSA (781.25 μg ml−1) (Table 3).
The NEs prepared using BAC as a surfactant exhibited positive effect on the bacterial inhibition capacity of LA, while the MIC value for BAC against both S. aureus and MRSA was 6.10 μg ml−1. BAC was selected as a surfactant because it is used as a preservative in pharmaceutical formulations and has antimicrobial activity on its own.21 MIC values for BAC have been reported to be 2–4 and 2–16 μg ml−1 against S. aureus and MRSA respectively.44 The most effective formulation in the BAC series was LA NE with a LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAC ratio of 1
BAC ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (% w/w), as evident by the lowest MIC value against both S. aureus and MRSA. The MIC values for this formulation were 3.05 and 1.52 μg ml−1 against S. aureus and MRSA respectively (Table 3), and were significantly lower (p < 0.05) than plain LA and BAC. This enhancement in antibacterial activity could be attributed to the combined antibacterial effect of LA and BAC, which acts by different modes of action. LA inhibits the FabI enzyme,23 and BAC exerts its activity by disruption and dissociation of cell membrane,45 thereby forming a nanoparticulate drug delivery system with a dual mechanisms of action. With these different modes of action of LA and BAC, as well as the intrinsic ability of nanosystems to overcome microbial resistance,46 the development of resistance to this nano system may not be possible for bacteria, as it would require multiple simultaneous gene mutations in the same microbial cell.7
1 (% w/w), as evident by the lowest MIC value against both S. aureus and MRSA. The MIC values for this formulation were 3.05 and 1.52 μg ml−1 against S. aureus and MRSA respectively (Table 3), and were significantly lower (p < 0.05) than plain LA and BAC. This enhancement in antibacterial activity could be attributed to the combined antibacterial effect of LA and BAC, which acts by different modes of action. LA inhibits the FabI enzyme,23 and BAC exerts its activity by disruption and dissociation of cell membrane,45 thereby forming a nanoparticulate drug delivery system with a dual mechanisms of action. With these different modes of action of LA and BAC, as well as the intrinsic ability of nanosystems to overcome microbial resistance,46 the development of resistance to this nano system may not be possible for bacteria, as it would require multiple simultaneous gene mutations in the same microbial cell.7
The transformation of LA into a nanosized delivery system, i.e. NE, therefore enhanced its antibacterial activity against both sensitive (S. aureus) and resistant (MRSA) strains. An increase in the surface area of LA by transforming it into nanodroplets might have contributed to better a interaction with the bacterial membrane.14 This enhanced antibacterial activity of LA thus further expands its applicability as a drug delivery system. Furthermore, this study has highlighted the critical role that surfactants play, not only in providing effective size and zeta potential, but also in affecting the antibacterial activity of the LA NE. More specifically, the LA NE with BAC enhanced antibacterial activity, while those with other surfactants reduced antibacterial activity.
Therefore, LA NEs prepared using BAC as a surfactant were considered as optimized, and further studies were performed using this formulation.
3.2 Characterization of optimized LA NEs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA and BAC exhibited pseudoplastic flow characteristics. Pseudoplastic behavior has also been reported for a small droplet sized benznidazole loaded soybean o/w emulsion.49 One cannot express the viscosity of a pseudoplastic material by any single value, as no part of the consistency curve is linear for a pseudoplastic material.50 The viscosity of LA NE decreased from 322.44 ± 8.72 to 92.74 ± 2.17 mPas, with an increase in the rate of shear from 1 to 100 s−1. The viscosity for LA NE with a 1
1 (w/w) ratio of LA and BAC exhibited pseudoplastic flow characteristics. Pseudoplastic behavior has also been reported for a small droplet sized benznidazole loaded soybean o/w emulsion.49 One cannot express the viscosity of a pseudoplastic material by any single value, as no part of the consistency curve is linear for a pseudoplastic material.50 The viscosity of LA NE decreased from 322.44 ± 8.72 to 92.74 ± 2.17 mPas, with an increase in the rate of shear from 1 to 100 s−1. The viscosity for LA NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA and BAC was 92.74 ± 2.17 mPas at a shear rate 100 s−1. The viscosity of LA NE decreased with an increase in the rate of shear, which could be due to the alignment of the long axes of normally disarranged LA molecules in the direction of flow, and the subsequent disorientation of the NE system, with the release of some associated solvents resulting in a lowering of the micellar concentration and size of dispersed molecules.50
1 (w/w) ratio of LA and BAC was 92.74 ± 2.17 mPas at a shear rate 100 s−1. The viscosity of LA NE decreased with an increase in the rate of shear, which could be due to the alignment of the long axes of normally disarranged LA molecules in the direction of flow, and the subsequent disorientation of the NE system, with the release of some associated solvents resulting in a lowering of the micellar concentration and size of dispersed molecules.503.2.5.1 Accelerated conditions. The results obtained for MGD, PI and ZP of NE before and after centrifugation are presented in Table 4. Analysis by t-tests followed, by a non-parametric Mann–Whitney test, displayed no significant change (p > 0.05) in the MGD, PI and ZP of the centrifuged NE compared to their respective counterpart before centrifugation (10
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) at 25 ± 2 °C for 30 min. These findings confirm the stability of the formulated LA NE.
000 rpm) at 25 ± 2 °C for 30 min. These findings confirm the stability of the formulated LA NE.
LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAC (w/w) BAC (w/w) |
Before centrifugation | After centrifugation | ||||
|---|---|---|---|---|---|---|
| MGD (nm) | PI | ZP (mV) | MGD (nm) | PI | ZP (mV) | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
151.3 ± 1.15 | 0.097 ± 0.010 | 28.2 ± 1.50 | 148.9 ± 2.01 | 0.101 ± 0.013 | 24.8 ± 1.01 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 0.5 |
136.8 ± 1.15 | 0.197 ± 0.035 | 36.5 ± 3.79 | 131.2 ± 1.53 | 0.198 ± 0.006 | 29.3 ± 6.05 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.75 0.75 |
94.27 ± 3.68 | 0.156 ± 0.028 | 39.9 ± 5.20 | 92.1 ± 1.79 | 0.155 ± 0.020 | 36.6 ± 1.44 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
75.14 ± 3.02 | 0.145 ± 0.011 | 45.7 ± 1.27 | 74.3 ± 0.35 | 0.163 ± 0.010 | 43.1 ± 1.72 |
3.2.5.2 Physical stability. The t-tests, followed by a non-parametric Mann–Whitney test, displayed no significant changes (p > 0.05) within the individual parameter groups for the various times at 4 °C studied compared to their respective controls. There were however very evident changes observed in the MGD, PI and ZP of the NE stored at RT at days 60 and 90 (Table 5). These findings are indicative of the poor stability of the LA NE with a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA and BAC stored at RT for a period greater than 30 days. LA NE stored at RT also showed visual changes, such as phase separation and an increase in turbidity. The results thereby confirm the selection of 4 °C as a preferred storage condition for the LA NE with a 1
1 (w/w) ratio of LA and BAC stored at RT for a period greater than 30 days. LA NE stored at RT also showed visual changes, such as phase separation and an increase in turbidity. The results thereby confirm the selection of 4 °C as a preferred storage condition for the LA NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio of LA and BAC.
1 (w/w) ratio of LA and BAC.
LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAC (w/w) BAC (w/w) |
Time (days) | MGD (nm) | PI | ZP (mV) | |||
|---|---|---|---|---|---|---|---|
| 4 °C | RT | 4 °C | RT | 4 °C | RT | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 75.14 ± 3.02 | 75.14 ± 3.02 | 0.145 ± 0.011 | 0.145 ± 0.011 | 45.7 ± 1.27 | 45.7 ± 1.27 |
| 30 | 84.22 ± 2.64 | 120.3 ± 0.51 | 0.186 ± 0.009 | 0.181 ± 0.007 | 45.2 ± 5.35 | 41.8 ± 8.80 | |
| 60 | 90.39 ± 1.99 | 267.6 ± 2.48 | 0.188 ± 0.064 | 0.336 ± 0.025 | 44.4 ± 3.30 | 30.6 ± 7.56 | |
| 90 | 91.3 ± 1.64 | 325 ± 8.21 | 0.199 ± 0.029 | 0.510 ± 0.183 | 45.8 ± 6.30 | 27.4 ± 6.29 | |
3.2.6.1 MIC determination. The LA NE with a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio had greater activity amongst all the formulations with MIC values of 3 μg ml−1 and 1.5 μg ml−1 against S. aureus and MRSA respectively (Table 3). Therefore, the LA NE with a 1
1 (w/w) ratio had greater activity amongst all the formulations with MIC values of 3 μg ml−1 and 1.5 μg ml−1 against S. aureus and MRSA respectively (Table 3). Therefore, the LA NE with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) ratio was considered to be optimized, and further microbiological evaluation studies were performed using this formulation.
1 (w/w) ratio was considered to be optimized, and further microbiological evaluation studies were performed using this formulation.
3.2.6.2 Fractional inhibitory concentration index (ΣFIC). The ΣFIC was calculated to determine whether synergy, indifference or antagonism had occurred by combining the LA and BAC in the NE. The FIC indices and their significance are represented in Table 6. The calculated ΣFIC for optimized LA NE formulation were 0.5 and 0.25 against S. aureus and MRSA (Table 6) respectively, which all indicated that the combination of LA and BAC had a synergistic effect (Table 1) on antibacterial activity.
LA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAC (w/w) BAC (w/w) |
ΣFIC | Results | ||
|---|---|---|---|---|
| S. aureus | MRSA | S. aureus | MRSA | |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 | 0.25 | Synergy | Synergy |
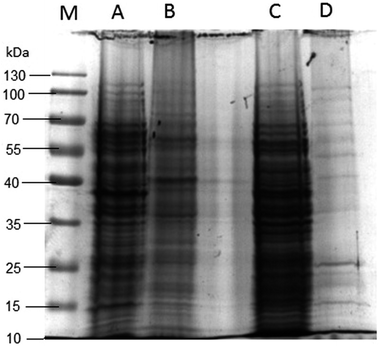
3.2.6.3 Bacterial protein degradation. Gel electrophoresis of the bacterial proteins was undertaken to determine the effect of the LA NE on cellular soluble proteins and the cell membrane of S. aureus and MRSA. Fig. 2 represents the gel electrophoresis pattern for the untreated and LA NE treated S. aureus and MRSA. The control bacterial cell protein electrophoresis bands of S. aureus and MRSA appeared strong and clear. After treating the bacteria with LA NE for 24 h, the bands of all molecular weight proteins for S. aureus and MRSA appeared faded compared to the control. The bands of all molecular weight proteins for S. aureus (Lane B, Fig. 2) appeared more faded than its control (Lane A, Fig. 2), although there was less fading for MRSA (Fig. 2C and D). This shows that the impact of the LA NE on the cell membrane damage of the MRSA was greater than that of S. aureus after treatment for 24 h. These results showed that the LA NE decreased the content of cellular soluble proteins by permeating and disrupting cell membranes, and are in good agreement with previous findings, where chitosan displayed the same results against P. aeruginosa.51 However, the mechanism of protein breakdown is unclear.
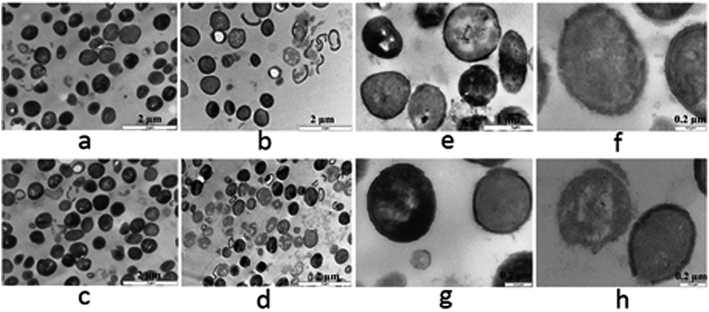
3.2.6.4 Bacterial cell morphology. The disruption of the bacterial cell membrane was further evaluated by assessing the morphological changes induced in S. aureus and MRSA cells after treatment with LA NE for 24 h. For this purpose, electron microscopic analysis was performed using TEM, as it is an important tool for researchers to assess the morphological changes in bacterial cells after treatment with the antibacterial agents.52 The TEM images showed that the control cells (Fig. 3a, c, e and g) exhibited a smooth and intact membrane without leakage of intracellular components and no prominent damage on the cell surface, while bacterial cells treated with LA NE (Fig. 3b, d, f and h) were damaged intensely. The closer view of LA NE treated S. aureus and MRSA cells (Fig. 3f and h) showed that it had a strong impact on the integrity of bacterial cell membrane, while the normal shape of bacterial cells was retained. The TEM images (Fig. 3) obtained also revealed that there was a strong effect of the LA NE on the MRSA as compared to S. aureus. Most of the MRSA cells were completely damaged, while there was cell membrane damage for S. aureus, although very few cells were completely damaged. These TEM analysis results corroborate well with the gel electrophoresis results and the MIC values for LA NE against both S. aureus and MRSA. From the TEM analysis, it was concluded that the cell membrane appears to be the main target of LA NE action due to the presence of BAC in the formulation,45 in addition to inhibiting FabI as one of the other plausible mechanism of action due to LA.23
| Entry | ELAS | Eint kcal mol−1 |
|---|---|---|
| 1 | ELA–BAC | −109.34 |
| 2 | ELA–SLS | −81.21 |
| 3 | ELA–Tween 80 | −35.01 |
| 4 | ELA–Lutrol F68 | −60.26 |
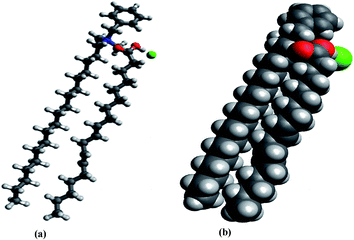
LA is a polyunsaturated carboxylic acid with an 18-carbon chain and two cis double bonds, and the cationic surface-acting agent BAC is a heterogeneous mixture of alkyl benzyl dimethyl ammonium chloride with an 18-carbon alkyl chain. In the NE system, the carboxylate group of LA molecules could have formed an ionic bond with the positively charged quaternary ammonium nitrogen of BAC molecule by Coulomb attraction without forming a covalent bond,53,54 which is clearly observed in the LA–BAC complex system (Fig. 4). The carboxylate group of LA is laid in between the chloride ion and quaternary ammonium nitrogen of BAC, which indicates the formation of a strong ionic bond between LA and BAC. Sarveiya et al. proved that the diffusion of drug molecules across a lipophilic membrane can be increased by the formation of ion-pairs.54,55 The formation of an LA ion pair with BAC (stable complex) in the NE may have facilitated its diffusion through the bacterial cell membrane. The enhanced penetration of LA along with the biocidal BAC into the bacterial cell might therefore have contributed to the increased antibacterial activity. In this system, it is proposed that the lipophilic hydrocarbon chains of LA and BAC formed the micellar hydrophobic central core, while the ion paired hydrophilic heads formed the hydrophilic surface of the micellar shell.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an in vitro cell culture system was used to determine the biological efficacy of optimized LA NE. Cytotoxicity evaluations were performed against Hep G2, MCF 7 and HeLa cells using the MTT assay. The cell viability within the concentration range studied (20 to 100 μg ml−1) was between 80.98 to 58.70% for Hep G2 cells, 77.21 to 64.25% for MCF 7 cells, and 78.51 to 57.92% for HeLa cells (Fig. 5). These findings displayed a dose dependent effect of the LA NE on cell viability, as a similar trend was observed across all cell lines. At a low concentration (20 μg ml−1), the LA NE (1
1, an in vitro cell culture system was used to determine the biological efficacy of optimized LA NE. Cytotoxicity evaluations were performed against Hep G2, MCF 7 and HeLa cells using the MTT assay. The cell viability within the concentration range studied (20 to 100 μg ml−1) was between 80.98 to 58.70% for Hep G2 cells, 77.21 to 64.25% for MCF 7 cells, and 78.51 to 57.92% for HeLa cells (Fig. 5). These findings displayed a dose dependent effect of the LA NE on cell viability, as a similar trend was observed across all cell lines. At a low concentration (20 μg ml−1), the LA NE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) showed minimal cell death, however, as the concentration of LA NE (1
1) showed minimal cell death, however, as the concentration of LA NE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) increased, the cell viability decreased significantly (p < 0.05). As the formulation studied contained equal amounts of LA and BAC, these results directly correlate with other studies that reported a dose dependent toxicity of BAC when studied against multiple cell lines.57,58 The results showed that LA NE, at a concentration of 20 μg ml−1, presented a cell viability of between 77.21 to 80.98% (Fig. 5), which is considered to be of low toxicity level.59 Interestingly, this formulation with a 1
1) increased, the cell viability decreased significantly (p < 0.05). As the formulation studied contained equal amounts of LA and BAC, these results directly correlate with other studies that reported a dose dependent toxicity of BAC when studied against multiple cell lines.57,58 The results showed that LA NE, at a concentration of 20 μg ml−1, presented a cell viability of between 77.21 to 80.98% (Fig. 5), which is considered to be of low toxicity level.59 Interestingly, this formulation with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio also had the greatest antimicrobial activity, with MIC values of 3.05 μg ml−1 and 1.52 μg ml−1 against S. aureus and MRSA respectively (Table 3).
1 ratio also had the greatest antimicrobial activity, with MIC values of 3.05 μg ml−1 and 1.52 μg ml−1 against S. aureus and MRSA respectively (Table 3).
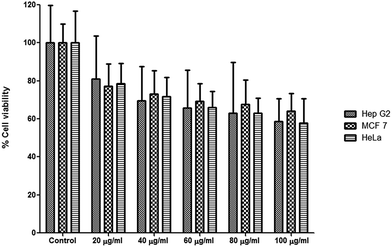 | ||
| Fig. 5 Cytotoxicity assay displaying percentage cell viability after exposure of cells (Hep G2, MCF-7 and HeLa) to optimized LA NE (n = 6). | ||
This data therefore suggests that the antibacterial dosage of LA NE against S. aureus and MRSA are well below the toxic dosage studied (20 μg ml−1), and could therefore be considered safe due to their nontoxicity to mammalian cells. The cytotoxicity of BAC to various mammalian cells is well documented in the literature58,60,61 with the highest tolerated dose against HepG2 cell lines reported as 1 μg ml−1.62,63 In this study, its cytotoxicity was reduced greatly in the presence of LA in the developed NE at the different concentration levels tested. This drastic lowering of the BAC cytotoxicity in combination with LA could be due to its ion pairing with oppositely charged LA molecules (as discussed under molecular modeling studies), and its partitioning into micelles. These effects collectively reduced the concentration of the free BAC molecules in the NE system. It has been reported that free BAC concentration in the NE is less due to its partitioning in the micelles.64 The optimized LA NE formulation therefore displays the ideal characteristics of an antimicrobial to be used for biomedical and pharmaceutical applications by displaying both good antimicrobial activity and non-toxicity against the mammalian cells studied.
4. Conclusion
Bacterial resistance to antibiotics has become an issue of global concern. In the past few years, nano based drug delivery systems have proven to be a promising approach to combat resistant bacterial infections. In this study, LA, which is an antibacterial material, was transformed into a stable NE using BAC as a stabilizing agent. The developed LA NE formulation with a MGD of below 100 nm exhibited enhanced antibacterial activity against susceptible and resistant S. aureus strains compared to plain LA. From the current investigation, it can be concluded that selecting the appropriate excipients to formulate NEs of non-drug fatty acids could enhance their antibacterial potential, a strategy that can be used to eradicate serious nosocomial infections where currently used antibacterial drugs are ineffective. Considering the slow rate of development of antibacterial drugs and the fast emerging superbugs, there is a need to develop such nanomaterials with multiple mechanisms of actions for biomedical and pharmaceutical applications.Acknowledgements
The authors thank University of KwaZulu-Natal (UKZN) and National Research Foundation of South Africa for financial support. Microscopy and Micro-analysis Unit (UKZN, Westville Campus) is acknowledged for the TEM analysis. We thank Anton Paar Southern Africa (Pty) Ltd for rheological analysis of the samples and Ms Carrin Martin for proof reading the manuscript.References
- I. R. Bell, G. E. Schwartz, N. N. Boyer, M. Koithan and A. J. Brooks, European Journal of Integrative Medicine, 2013, 5, 126–140 CrossRef PubMed.
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- R. M. Klevens, J. R. Edwards, F. C. Tenover, L. C. McDonald, T. Horan and R. Gaynes, Clin. Infect. Dis., 2006, 42, 389–391 CrossRef PubMed.
- H. S. Gold and R. C. Moellering, N. Engl. J. Med., 1996, 335, 1445–1453 CrossRef CAS PubMed.
- L. B. Rice, Curr. Opin. Microbiol., 2009, 12, 476–481 CrossRef CAS PubMed.
- C. Beaulac, S. Clement-Major, J. Hawari and J. Lagacé, Antimicrob. Agents Chemother., 1996, 40, 665–669 CAS.
- R. S. Kalhapure, C. Mocktar, D. R. Sikwal, S. J. Sonawane, M. K. Kathiravan, A. Skelton and T. Govender, Colloids Surf., B, 2014, 117, 303–311 CrossRef CAS PubMed.
- K. Blecher, A. Nasir and A. Friedman, Virulence, 2011, 2, 395–401 CrossRef PubMed.
- L. Zhang, D. Pornpattananangkul, C.-M. Hu and C.-M. Huang, Curr. Med. Chem., 2010, 17, 585–594 CrossRef CAS.
- R. S. Kalhapure, N. Suleman, C. Mocktar, N. Seedat and T. Govender, J. Pharm. Sci., 2015, 104, 872–905 CrossRef CAS PubMed.
- A. Sharma, D. Kumar Arya, M. Dua, G. S. Chhatwal and A. K. Johri, Expert Opin. Drug Delivery, 2012, 9, 1325–1332 CrossRef CAS PubMed.
- D. J. McClements, Soft Matter, 2011, 7, 2297–2316 RSC.
- W. Li, H. Chen, Z. He, C. Han, S. Liu and Y. Li, LWT--Food Sci. Technol., 2015, 62, 39–47 CrossRef CAS PubMed.
- S. Sugumar, V. Ghosh, M. J. Nirmala, A. Mukherjee and N. Chandrasekaran, Ultrason. Sonochem., 2014, 21, 1044–1049 CrossRef CAS PubMed.
- V. Ghosh, A. Mukherjee and N. Chandrasekaran, Ultrason. Sonochem., 2013, 20, 338–344 CrossRef CAS PubMed.
- J. Pannu, A. McCarthy, A. Martin, T. Hamouda, S. Ciotti, L. Ma, J. Sutcliffe and J. Baker, Antimicrob. Agents Chemother., 2011, 55, 4211–4217 CrossRef CAS PubMed.
- A. Azeem, M. Rizwan, F. J. Ahmad, Z. Iqbal, R. K. Khar, M. Aqil and S. Talegaonkar, AAPS PharmSciTech, 2009, 10, 69–76 CrossRef CAS PubMed.
- T. Kommuru, B. Gurley, M. Khan and I. Reddy, Int. J. Pharm., 2001, 212, 233–246 CrossRef CAS.
- K. Ziani, Y. Chang, L. McLandsborough and D. J. McClements, J. Agric. Food Chem., 2011, 59, 6247–6255 CrossRef CAS PubMed.
- R. S. Kalhapure and K. G. Akamanchi, J. Surfactants Deterg., 2015, 18, 537–545 CrossRef CAS.
- M. S. Karabit, O. T. Juneskans and P. Lundgren, Int. J. Pharm., 1988, 46, 141–147 CrossRef CAS.
- J. E. Kinsella, K. S. Broughton and J. W. Whelan, J. Nutr. Biochem., 1990, 1, 123–141 CrossRef CAS.
- C. J. Zheng, J.-S. Yoo, T.-G. Lee, H.-Y. Cho, Y.-H. Kim and W.-G. Kim, FEBS Lett., 2005, 579, 5157–5162 CrossRef CAS PubMed.
- A. P. Desbois and V. J. Smith, Appl. Microbiol. Biotechnol., 2010, 85, 1629–1642 CrossRef CAS PubMed.
- P. A. Smith and F. E. Romesberg, Nat. Chem. Biol., 2007, 3, 549–556 CrossRef CAS PubMed.
- R. Schiffelers, G. Storm and I. Bakker-Woudenberg, J. Antimicrob. Chemother., 2001, 48, 333–344 CrossRef CAS PubMed.
- Y. Shigeta, H. Imanaka, H. Ando, A. Ryu, N. Oku, N. Baba and T. Makino, Biol. Pharm. Bull., 2004, 27, 591–594 CAS.
- F. Sainsbury, B. Zeng and A. P. Middelberg, Curr. Opin. Chem. Eng., 2014, 4, 11–17 CrossRef PubMed.
- C. Lovelyn and A. A. Attama, J. Biomater. Nanobiotechnol., 2011, 2, 626–639 CrossRef CAS.
- M. Botelho, J. Dent., 2000, 28, 565–570 CrossRef CAS.
- N. Suleman, R. S. Kalhapure, C. Mocktar, S. Rambharose, M. Singh and T. Govender, RSC Adv., 2015, 5, 34967–34978 RSC.
- M. Z. Sitohy, S. A. Mahgoub and A. O. Osman, Int. J. Food Microbiol., 2012, 154, 19–29 CrossRef CAS PubMed.
- C. Wang, T. Chang, H. Yang and M. Cui, Food Control, 2015, 47, 231–236 CrossRef CAS PubMed.
- C.-Y. Wang, C.-P. Hsu, H.-W. Huang and B. B. Yang, Food Res. Int., 2013, 54, 1482–1487 CrossRef CAS PubMed.
- T. A. Halgren, J. Comput. Chem., 1996, 17, 490–519 CrossRef CAS.
- W. D. Harkins, Proc. Natl. Acad. Sci. U. S. A., 1919, 5, 539–546 CrossRef CAS.
- B. Y. Yu, J. W. Chung and S.-Y. Kwak, Environ. Sci. Technol., 2008, 42, 7522–7527 CrossRef CAS.
- L. Tomar, C. Tyagi, P. Kumar, Y. E. Choonara, L. Du Toit and V. Pillay, Int. J. Pharmacol. Pharmaceut. Tech., 2012, 1, 62–67 Search PubMed.
- S. Rambharose, R. S. Kalhapure, K. G. Akamanchi and T. Govender, J. Mater. Chem. B, 2015, 3, 6662–6675 RSC.
- M. R. Zahi, H. Liang and Q. Yuan, Food Control, 2015, 50, 554–559 CrossRef CAS PubMed.
- S. Savic, S. Tamburic and M. M. Savic, Expert Opin. Drug Delivery, 2010, 7, 353–369 CrossRef CAS PubMed.
- H. Choudhury, B. Gorain, S. Karmakar, E. Biswas, G. Dey, R. Barik, M. Mandal and T. K. Pal, Int. J. Pharm., 2014, 460, 131–143 CrossRef CAS PubMed.
- D. Krsta, C. Ku, I. T. Crosby, B. Capuano and D. T. Manallack, Anti-Infect. Agents, 2014, 12, 80–84 CrossRef CAS.
- C. Raggi, P. Filippini, M. Monaco, A. Pantosti, R. Creti and L. Baldassarri, Clin. Microbiol.: Open Access, 2013, 2, 1–6 Search PubMed.
- G. McDonnell and A. D. Russell, Clin. Microbiol. Rev., 1999, 12, 147–179 CAS.
- R. Y. Pelgrift and A. J. Friedman, Adv. Drug Delivery Rev., 2013, 65, 1803–1815 CrossRef CAS PubMed.
- D. J. McClements, Curr. Opin. Colloid Interface Sci., 2002, 7, 451–455 CrossRef CAS.
- D. J. McClements, Adv. Colloid Interface Sci., 2002, 97, 63–89 CrossRef CAS.
- L. Streck, M. M. de Araújo, I. de Souza, M. F. Fernandes-Pedrosa, E. S. T. do Egito, A. G. de Oliveira and A. A. da Silva-Júnior, J. Mol. Liq., 2014, 196, 178–186 CrossRef CAS PubMed.
- A. Martin, J. Swarbrick and A. Cammarata, Physical pharmacy: physical chemical principles in the pharmaceutical sciences, Lea and Febiger, Philadelphia, 3rd edn, 1983, ch. 19, pp. 524–526 Search PubMed.
- Y. Tao, L.-H. Qian and J. Xie, Carbohydr. Polym., 2011, 86, 969–974 CrossRef CAS PubMed.
- K. Xing, X. G. Chen, M. Kong, C. S. Liu, D. S. Cha and H. J. Park, Carbohydr. Polym., 2009, 76, 17–22 CrossRef CAS PubMed.
- D. Woodward and G. Ambrus, U.S. Pat., Appl. US 2002/0198209 A1, 2001, pp. 1–11.
- V. Sarveiya, J. F. Templeton and H. A. Benson, J. Pharm. Pharmacol., 2004, 56, 717–724 CrossRef CAS PubMed.
- V. Sarveiya, J. F. Templeton and H. A. Benson, Eur. J. Pharm. Sci., 2005, 26, 39–46 CrossRef CAS PubMed.
- G. Oberdörster, A. Maynard, K. Donaldson, V. Castranova, J. Fitzpatrick, K. Ausman, J. Carter, B. Karn, W. Kreyling and D. Lai, Part. Fibre Toxicol., 2005, 2, 8 CrossRef PubMed.
- C. Chang, A. Q. Zhang, D. B. Kagan, H. Liu and C. M. Hutnik, Clin. Exp. Ophthalmol., 2014, 43, 164–172 Search PubMed.
- A. Iwasawa, M. Ayaki and Y. Niwano, Regul. Toxicol. Pharmacol., 2013, 66, 177–183 CrossRef CAS PubMed.
- X. Cao, C. Cheng, Y. Ma and C. Zhao, J. Mater. Sci.: Mater. Med., 2010, 21, 2861–2868 CrossRef CAS PubMed.
- T. Deutschle, U. Porkert, R. Reiter, T. Keck and H. Riechelmann, Toxicol. In Vitro, 2006, 20, 1472–1477 CrossRef CAS PubMed.
- C. Chang, A. Q. Zhang, D. B. Kagan, H. Liu and C. M. Hutnik, Clin. Exp. Ophthalmol., 2015, 43, 164–172 Search PubMed.
- E. Borenfreund and O. Borrero, Cell Biol. Toxicol., 1984, 1, 55–65 CrossRef CAS.
- E. Borenfreund and C. Shopsis, Xenobiotica, 1985, 15, 705–711 CrossRef CAS PubMed.
- J. Liu, G. W. Lu, M. Sandoval, Y. Ciringh, G. Xue, D. Jaeger, K. Kompanik, J. Jiao and K. M. Gelotte, AAPS PharmSciTech, 2009, 10, 1216–1223 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |