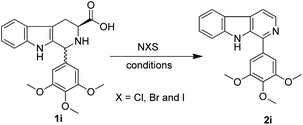
An efficient one-pot decarboxylative aromatization of tetrahydro-β-carbolines by using N-chlorosuccinimide: total synthesis of norharmane, harmane and eudistomins†
Ahmed Kamal*abc,
Manda Sathisha,
A. V. G. Prasanthib,
Jadala Chetnab,
Yellaiah Tangellaa,
Vunnam Srinivasulua,
Nagula Shankaraiahb and
Abdullah Alarific
aMedicinal Chemistry & Pharmacology, CSIR-Indian Institute of Chemical Technology, Hyderabad 500 007, India. E-mail: ahmedkamal@iict.res.in
bDepartment of Medicinal Chemistry, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad-500 037, India
cCatalytic Chemistry Research Chair, Chemistry Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia
First published on 15th October 2015
Abstract
A facile method for the synthesis of a variety of β-carbolines and their natural products such as norharmane (2a), harmane (2b), eudistomins I, N, T, and U (6, 7, 9 and 10, respectively) has been successfully developed via a decorboxylative aromatization tool by employing N-chlorosuccinimide (NCS) as a mild and efficient reagent. Gratifyingly, this reagent system proceeds in a one-pot manner and converted all the tetrahydro-β-carboline acids into their corresponding decorboxylative aromatic products with good to excellent yields. Additionally, this system works well in the case of tetrahydro-β-carboline esters to produce their aromatic partners in high yields.
Introduction
It is well-known that β-carboline alkaloids having a tricyclic planar ring system are found in various synthetic/natural indole alkaloids.1 This class of compounds (norharmane, harmane and harmine) are originally isolated from Peganum harmala and a group of β-carbolines,2 termed as eudistomins (eudistomine I, N, T and U) are isolated from ascidians.3,4 Most of these alkaloids exhibit antibacterial,5 antimalarial,6 antitumor,7anti-HIV8 activities, and also show binding affinity towards 5-hydroxy serotonin receptors,9 monoamine oxidase10 and benzodiazepine receptors11 in the central nervous system. In light of these medicinal properties, there is a need for the development of mild and efficient methods towards the synthesis of β-carboline and their natural products. In recent years, we have been involved in the total synthesis of several natural/unnatural β-carboline alkaloids such as arborescidine alkaloids, quinolactacin B, PDE5 inhibitor, etc.12 Moreover, the β-carboline ring system has also been used as versatile synthetic intermediates in the development of pharmaceutically useful motifs.13The decarboxylative aromatization is one of the most attractive transformations in the organic synthesis.14 However, decarboxylative aromatization of tetrahydro β-carbolines that involves C–H bond formation has received considerably less attention. A common method for the decarboxylative aromatization employs transition-metal oxidants at higher temperatures.15,16 In the past, this transformation was achieved by using K2Cr2O7 (ref. 16) in acetic acid under heating for prolonged reaction times in presence of persulfate employing catalytic silver (Ag+/S2O8−).17 Generally, Pictet–Spengler reaction followed by decarboxylative aromatization is the most widely studied method to access β-carbolines.18 On the other hand, aromatization of the tetrahydro-β-carboline carboxylate is reported to be carried out by using mild oxidants like MnO2,19 SeO2,20 palladium on carbon21 and sulfur22 with excess usage of the oxidants as well as prolonged reaction time. Other organic oxidants like DDQ23 and chloranil24 were also used for aromatization but the yields are not satisfactory. However, most of these methods require expensive reagents and drastic reaction conditions that lack environmental safety. Hence, the development of inexpensive, operationally simple and environmentally safe protocols that could be carried out under milder reaction conditions are valuable.
Next, N-halosuccinimides (NXS, X = Cl, Br and I) are considered as halogenating and mild oxidizing agents that can be used as a source of halogen in radical reactions for various electrophilic additions25,26 as well as for mild oxidations.27 Earlier, Roy and co-workers have reported the decarboxylative halogenation of α,β-unsaturated carboxylic acids by using NXS.28 Later, Kuang and co-workers have reported the stereoselective decarboxylative bromination of α,β-unsaturated carboxylic acids by using NBS and lithium acetate under microwave conditions.29 However, N-chlorosuccinimide (NCS) is a versatile and cost effective reagent for the mild oxidation as well as halogenation, having lesser toxicity than other oxidants.30 Moreover, the by-product succinimide is highly soluble in water (1 g/3 mL at 25 °C),31 easy to remove from reaction mixture during the work-up and also in large-scale reactions the succinimide from aqueous phase can be converted into the starting reagent N-chlorosuccinimide by treating with the sodium hypochlorite to make the method sustainable.32 Recently, trichloroisocyanuric acid (TCCA) is also being used for aromatization of tetrahydro-β-carboline esters,33 but the draw-back of this reagent is solubility of its by-product cyanuric acid (0.27 g/100 mL at 25 °C) in water. Based on these observations, we were prompted to look for a straightforward, operationally simple and high yielding protocol for the synthesis of β-carbolines using N-chlorosuccinimide. Therefore, we herein report a new one-pot decarboxylative aromatization tandem aromatization strategy for the synthesis of several β-carbolines and their alkaloids such as norharmane (2a), harmane (2b), eudistomin I (6), eudistomin N (7), eudistomin T (9) and eudistomin U (10) in excellent yields. In addition, this method was also successfully applied for the aromatization of tetrahydro-β-carboline ester into their corresponding β-carboline esters in high yields.
Results and discussion
In this study, we have initially tested the feasibility of the reaction of 1i with N-halosuccinimides (NXS, X = Cl, Br and I, 1.5 equiv.) in the presence of TEA (2 equiv.) and THF as a solvent at ambient temperature for 12 h. It was observed that the product 2i obtained in moderate yields 60%, 53% and 48% (entry 1, 2, 3, Table 1) with different reagents. Later, when we enhanced the stoichiometric ratio of NXS (2.1 equiv.) and TEA (2.5 equiv.), the desired products were obtained in good yields 85%, 78% and 70% (entry 4, 5, 6, Table 1) respectively. Gratifyingly, the starting materials were completely consumed and even the reaction time was also decreased to 30 min. However, increase in the stoichiometric ratio of NXS (3 equiv.) reagent had no effect on yields and even the chlorinated product was not observed in this reaction process. Based on these model experiments, 2.1 equiv. of NCS was found to be slightly superior in comparison to NBS or NIS, and as well cost effective. The effect of solvent on the reaction was also studied by using various solvents such as THF, CH3CN, 1,4-dioxane, DMF, DMSO, CH2Cl2 and it was observed that the product was obtained in 30 min with higher yields in the case of DMF as solvent (Table 1).| Entry | NXS | Equiv. | Time (h) | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Tetrahydro-β-carboline acid (1i, 1 equiv.), NXS and TEA.b Isolated yield.c Starting material was recovered. | |||||
| 1 | NCS | 1.5 | 12 | THF | 60c |
| 2 | NBS | 1.5 | 12 | THF | 53c |
| 3 | NIS | 1.5 | 12 | THF | 48c |
| 4 | NCS | 2.1 | 1 | THF | 85 |
| 5 | NBS | 2.1 | 1 | THF | 78 |
| 6 | NIS | 2.1 | 1 | THF | 70 |
| 7 | NCS | 3.0 | 1 | THF | 85 |
| 8 | NCS | 2.1 | 1 | Acetonitrile | 65 |
| 9 | NCS | 2.1 | 1 | 1,4-Dioxane | 71 |
| 10 | NCS | 2.1 | 0.5 | DMF | 92 |
| 11 | NCS | 2.1 | 1 | DMSO | 88 |
| 12 | NCS | 2.1 | 1 | DCM | 68 |
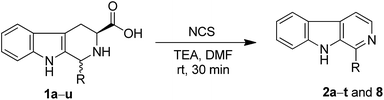
After establishing optimal conditions, we studied the reactivity of various tetrahydro-β-carboline acids differing at position-1 and the results are shown in Table 2. The substrates tetrahydro-β-carboline acids successfully synthesized by Pictet–Spengler reaction of L-tryptophan and substituted benzaldehydes. Typically, the substituents on tetrahydro-β-carboline acids slightly affected the yields, wherein good yields are observed in case of aromatic and heteroaromatic rings at position-1 compared to aliphatic substitutions (entry 1, 2, 3 and 4, Table 2). From Table 2, it is observed that the nature of substituents at position-1 considerably influence the yields of the products. For example, substrates with electron-rich groups afforded noticeably high yields compared to electron-poor groups (entry 14 and 15, Table 2). Interestingly, the tetrahydro-β-carboline acids with fused ring systems like naphthyl and phenanthryl groups also provided excellent yields (entry 17 and 18, Table 2), however moderate yields are observed in case of substrates with phenoxy substitutions at position-1 (entry 10 and 11, Table 2). Moreover, the natural β-carboline alkaloids norharmane (2a) and harmane (2b) are synthesized successfully by using this protocol with appreciable yields (entries 1 and 2, Table 2).
| Entry | R | Product | Yieldb (%) |
|---|---|---|---|
| a 1 (1 mmol) in DMF, NCS (2.1 equiv.) and TEA (2.5 equiv.) stirred for 30 min at rt.b Isolated yield. | |||
| 1 | H | Norharmane (2a) | 80 |
| 2 | Me | Harmane (2b) | 82 |
| 3 | n-Propyl | 2c | 78 |
| 4 | 4-Methoxybenzyl | 2d | 90 |
| 5 | Phenyl | 2e | 85 |
| 6 | p-Tolyl | 2f | 88 |
| 7 | p-Methoxy phenyl | 2g | 93 |
| 8 | 3,4,5-Trimethoxy phenyl | 2h | 92 |
| 9 | 4-Hydroxy-3-methoxy phenyl | 2i | 83 |
| 10 | 3-Hydroxyphenyl | 2j | 78 |
| 11 | 4-Chlorophenyl | 2k | 80 |
| 12 | 4-Fluorophenyl | 2l | 83 |
| 13 | 4-Cyanophenyl | 2m | 81 |
| 14 | 4-Nitrophenyl | 2n | 79 |
| 15 | Piperonyl | 2o | 91 |
| 16 | Naphthyl | 2p | 94 |
| 17 | 9-Phenanthryl | 2q | 95 |
| 18 | 3-Pyridyl | 2r | 91 |
| 19 | 4-Fluoro-3-methoxyphenyl | 2s | 88 |
| 20 | 4-Trifluorotolyl | 2t | 85 |
| 21 | N-Boc-2-phenethylamino | 8 | 80 |
Inspired by the above results, we were prompted to apply this method for the synthesis of marine β-carboline alkaloids like eudistomins I, N, T and U. Eudistomin I, N and T were isolated from Caribbean tunicate Eudistoma Olivaceum and shows strong antiviral and antimicrobial activities.3b,34 In 1987, the total synthesis of eudistomin I was reported,3b,35 while Rinehart and co-workers in order to characterize the alkaloid isolated eudistomin N, described the synthesis of this bromo-β-carbolines.3b,36 Moreover, eudistomin N was found to be the most potent antiviral agent even compared to harmine.37 Whereas, eudistomin T was isolated in 1987 by Kinzer and Cardellina II,38 another marine β-carboline alkaloid eudistomin U was isolated from the marine ascidian Lissoclinum fragile and was reported to posses antimicrobial as well as anticancer activities with strong DNA binding potential.4 Both these eudistomins T and U succumbed to their total synthesis six times.39,40 Overall, from the literature it was observed that the syntheses of such β-carbolines rarely involve decarboxylative aromatization and often utilized either metal catalysts, drastic conditions or prolonged reaction times and with lower yields to construct the core skeleton. In sight of these aspects, we were driven to synthesize these natural β-carbolines from the commercially available starting materials which could be a efficient route with higher yields than the previously reported syntheses.
Initially, we started the total synthesis of eudistomin I from Pictet–Spengler reaction of L-tryptophan (1) and N-boc prolinal to provide the corresponding N-boc-pyrrolidinyl tetrahydro-β-carboline acid, which was then directly used for further deprotection of boc group using 2 N HCl in 1,4-dioxane to afford the hydrochloride salt 5. Salt 5 was produced the eudistomin I (6) under optimized reaction conditions by using NCS in 70% yield. Interestingly, it was observed that decarboxylation, aromatization and imine formation were successfully achieved in a one-pot manner (Scheme 1).
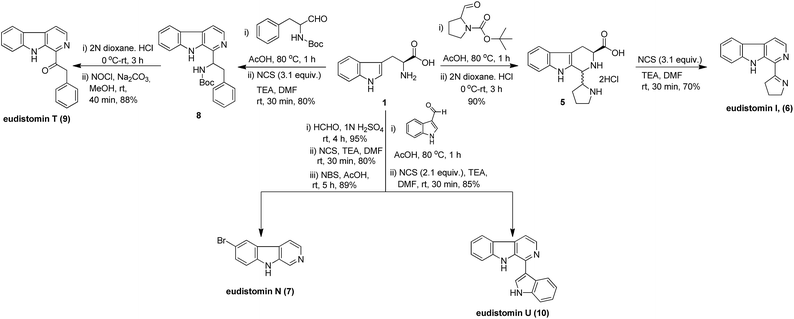 | ||
| Scheme 1 Total synthesis of eudistomin I (6), eudistomin N (7), eudistomin T (9) and eudistomin U (10) by using NCS. | ||
Next, the synthesis of eudistomin N (7) was achieved by the Pictet–Spengler reaction of L-tryptophan (1) and formaldehyde followed by decarboxylative aromatization under optimized reaction conditions with NCS to afford norharmane (2a). The bromination of 2a with NBS in acetic acid afforded the eudistomin N (7) and it is important to note that the overall yield is considerably higher (91%, Scheme 1).
Subsequently, the total synthesis of eudistomin T (9) was also carried out with the acid catalyzed Pictet–Spengler condensation of L-tryptophan with N-boc-phenylalaninal and further reaction with NCS provided compound 8. This compound (8) was taken for deprotection of boc group with 2 N HCl in 1,4-dioxane to afford the corresponding amine, which was subsequently treated directly with sodium hypochlorite to produce eudistomin T (9, 88%, Scheme 1).
Finally, we also carried out the total synthesis of eudistomin U (10) with commercially available L-tryptophan. The Pictet–Spengler reaction of L-tryptophan and indole-3-carbaldehyde followed by reaction with NCS under optimized conditions produced eudistomin U (10) in good yield (85%, Scheme 1).
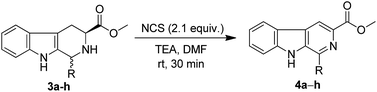
Additionally, to find out the scope of this protocol on the tetrahydro-β-carboline esters, we have reacted various substituted tetrahydro-β-carboline esters with NCS. The substrates β-carboline esters were synthesized in two steps from commercially available L-tryptophan methyl ester hydrochloride. Interestingly, all these substrates successfully yielded their corresponding aromatic partners (4a–h) in excellent yields (Table 3) with out losing ester group.
| Entry | R | Product | Yieldb (%) |
|---|---|---|---|
| a 3 (1 mmol) in DMF, NCS (2.1 equiv.) and TEA (2.5 equiv.) stirred for 30 min at rt.b Isolated yield. | |||
| 1 | 2-Phenethyl | 4a | 81 |
| 2 | 3,4,5-Trimethoxy phenyl | 4b | 94 |
| 3 | 1-Naphthyl | 4c | 95 |
| 4 | 4-Chlorophenyl | 4d | 92 |
| 5 | 9-Phenanthryl | 4e | 96 |
| 6 | 4-Fluoro-3-methoxy phenyl | 4f | 94 |
| 7 | 4-Trifluorotolyl | 4g | 91 |
| 8 | 1-N-Boc-2-phenethyl | 4h | 88 |
Further, to understand the mechanism a test reaction was performed on β-carboline acid (11, Fig. 1) under optimal conditions. It was observed that even after 48 h, the starting material was remained; it proves the sequence of decarboxylation followed by aromatization. Next, a reaction of tetrahydro-β-carbolines (12 and 13) under optimal reaction conditions provided partially dehydrogenated products (14 and 15) after 5 min, however fully aromatized β-carbolines (2g and 2n, Fig. 1) were observed after 12 h. In addition, we saw that the tetrahydro-β-carboline esters (3a-h) underwent aromatization only in 30 min. From all these results/findings, it was observed that the ester/acid functionality accelerates the reaction and the mechanism was proposed (Fig. 1).
Conclusion
In conclusion, we have developed an operationally simple, mild and efficient protocol for the one-pot decarboxylative aromatization of tetrahydro-β-carboline-3-carboxylic acids to the β-carbolines. This protocol employs a cost effective oxidant N-chlorosuccinimide that provides higher yields. This method could utilize an alternative to the traditional heating methods employing harsh metal-based reagents. Moreover, the efficacy of this reagent system was established for the total synthesis of biologically active some of the β-carbolines norharmane, harmane and marine β-carboline alkaloids eudistomin I, N, T and U (2a, 2b, 6, 7, 9 and 10) respectively. Additionally, aromatization of tetrahydro-β-carboline esters to the corresponding β-carboline esters are also achieved successfully in excellent yields.Acknowledgements
The authors, M. S, Y. T and V. S acknowledge the Council of Scientific and Industrial Research (CSIR), New Delhi (India) for the financial support under the 12th Five Year Plan project “Affordable Cancer Therapeutics (ACT)” (CSC0301). This project was also supported by King Saud University, Deanship of Scientific Research, Research Chair.References
- (a) R. Cao, W. Peng, Z. Wang and A. Xu, Curr. Med. Chem., 2007, 14, 479 CrossRef CAS; (b) W. D. Inman, B. W. M. Ray, N. C. Gassner, R. S. Lokey, K. Tenney, Y. Y. Shen, K. Tendyke, T. Suh and P. Crews, J. Nat. Prod., 2010, 73, 255 CrossRef CAS PubMed.
- (a) M. Moloudizargari, P. Mikaili, S. Aghajanshakeri, M. H. Asghari and J. Shayegh, Pharmacogn. Rev., 2013, 7, 199 CrossRef PubMed; (b) K. Patel, M. Gadewar, R. Tripathi, S. K. Prasad and D. K. Patel, Asian Pac. J. Trop. Biomed., 2012, 2, 660 CrossRef CAS.
- (a) R. A. Davis, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 1998, 61, 959 CrossRef CAS PubMed; (b) K. L. Rinehart Jr, J. Kobayashi, G. C. Harbour, J. Gilmore, M. Mascal, T. G. Holt, L. S. Shield and F. Lafargue, J. Am. Chem. Soc., 1987, 109, 3378 CrossRef.
- (a) K. F. Kinzer and J. H. Cardellina II, Tetrahedron Lett., 1987, 28, 925 CrossRef CAS; (b) A. Badre, A. Boulanger, E. Abou-Mansour, B. Banaigs, G. Combaut and C. Francisco, J. Nat. Prod., 1994, 57, 528 CrossRef CAS.
- (a) H. J. Shin, H. S. Lee and D. S. Lee, J. Microbiol. Biotechnol., 2010, 20, 501 CAS; (b) R. B. Volk and F. H. Furkert, Microbiol. Res., 2006, 161, 180 CrossRef CAS PubMed.
- (a) A. G. Shilabin, N. Kasanah, B. L. Tekwani and M. T. Hamann, J. Nat. Prod., 2008, 71, 1218 CrossRef CAS PubMed; (b) J. D. Winkler, A. T. Londregan and M. T. Hamann, Org. Lett., 2006, 8, 2591 CrossRef CAS PubMed; (c) Y. Boursereau and I. Coldham, Bioorg. Med. Chem. Lett., 2004, 14, 5841 CrossRef CAS PubMed.
- (a) M. R. Prinsep, J. W. Blunt and M. H. G. Munro, J. Nat. Prod., 1991, 54, 1068 CrossRef CAS; (b) A. Kamal, V. Srinivasulu, V. L. Nayak, M. Sathish, N. Shankaraiah, C. Bagul, N. V. Reddy, N. Rangaraj and N. Nagesh, ChemMedChem, 2014, 9, 2084 CrossRef CAS PubMed; (c) N. Shankaraiah, K. P. Siraj, S. Nekkanti, V. Srinivasulu, P. Sharma, K. R. Senwar, M. Sathish, M. V. Vishnuvardhan, S. Ramakrishna, C. Jadala, N. Nagesh and A. Kamal, Bioorg. Chem., 2015, 59, 130 CrossRef CAS PubMed; (d) N. Shankaraiah, S. Nekkanti, K. J. Chudasama, K. R. Senwar, P. Sharma, M. K. Jeengar, V. G. Naidu, V. Srinivasulu, G. Srinivasulu and A. Kamal, Bioorg. Med. Chem. Lett., 2014, 24, 5413 CrossRef CAS PubMed.
- (a) J. G. Tang, Y. H. Wang, R. R. Wang, Z. J. Dong, L. M. Yang, Y. T. Zheng and J. K. Liu, Chem. Biodiversity, 2008, 5, 447 CrossRef CAS PubMed; (b) X. Yu, W. Lin, J. Li and M. Yang, Bioorg. Med. Chem. Lett., 2004, 14, 3127 CAS.
- (a) B. Grella, M. Teitler, C. Smith, K. Herrick-Davis and R. A. Glennon, Bioorg. Med. Chem. Lett., 2003, 13, 4421 CrossRef CAS PubMed; (b) J. E. Audia, D. A. Evrard, G. R. Murdoch, J. J. Droste, J. S. Nissen, K. W. Schenck, P. Fludzinski, V. L. Lucaites, D. L. Nelson and M. L. Cohen, J. Med. Chem., 1996, 39, 2773 CrossRef CAS PubMed; (c) B. Grella, M. Dukat, R. Young, M. Teitler, K. Herrick-Davis, C. B. Gauthierr and R. A. Glennon, Drug Alcohol Depend., 1998, 50, 99 CrossRef CAS; (d) R. A. Glennon, M. Dukat, B. Grella, S. S. Hong, L. Costantino, M. Teitler, C. Smith, C. Egan, K. Davis and M. V. Mattson, Drug Alcohol Depend., 2000, 60, 121 CrossRef CAS.
- Q. Wu, R. Cao, M. Feng, X. Guan, C. Ma, J. Liu, H. Song and W. Peng, Med. Chem., 2009, 44, 533 CrossRef CAS PubMed.
- (a) T. J. Hagen, P. Skolnick and J. M. Cook, J. Med. Chem., 1987, 30, 750 CrossRef CAS; (b) W. E. Muller, K. J. Fehske, H. O. Borbe, U. Wollert, C. Nanz and H. Rommelspacher, Pharmacol., Biochem. Behav., 1981, 14, 693 CrossRef CAS; (c) S. P. Hollinshead, M. L. Trudell, P. Skolnick and J. M. Cook, J. Med. Chem., 1990, 33, 1062 CrossRef CAS.
- (a) A. Kamal, Y. Tangella, K. L. Manasa, M. Sathish, V. Srinivasulu, J. Chetna and A. Alarifi, Org. Biomol. Chem., 2015, 13, 8652 RSC; (b) W. A. Silva, M. T. Rodrigues, N. Shankaraiah, R. B. Ferreira, C. K. Z. Andrade, R. A. Pilli and L. S. Santos, Org. Lett., 2009, 11, 3238 CrossRef PubMed; (c) A. Kamal, M. K. Reddy, T. S. Reddy, L. S. Santos and N. Shankaraiah, Synlett, 2011, 61 CrossRef CAS; (d) N. Shankaraiah and L. S. Santos, Tetrahedron Lett., 2009, 50, 520 CrossRef CAS PubMed; (e) N. Shankaraiah, W. A. Silva, C. K. Z. Andrade and L. S. Santos, Tetrahedron Lett., 2008, 49, 4289 CrossRef CAS PubMed.
- (a) V. G. Erniest, X. Wang, N. D. Kimpe and A. Padwa, J. Org. Chem., 2010, 75, 424 CrossRef PubMed; (b) I. T. Raheem, P. S. Thiara, P. E. Aeterson and E. N. Jacobsen, J. Am. Chem. Soc., 2007, 129, 13404 CrossRef CAS PubMed; (c) M. S. Taylor, N. Tokunaga and E. N. Jacobsen, Angew. Chem., Int. Ed., 2005, 44, 6700 CrossRef CAS PubMed.
- (a) S. K. Adeniyi-jones and M. L. Karnovsky, J. Clin. Invest., 1981, 68, 365 CrossRef CAS; (b) E. V. Bellale, S. N. Huddar, U. S. Mahajan and K. G. Akamanchi, Pure Appl. Chem., 2011, 83, 607 CrossRef CAS.
- M. Ramu, M. M. Nizam and M. S. Mahsufi, Synlett, 2014, 25, 120 Search PubMed.
- G. Zhang, R. Cao, L. Guo, Q. Ma, W. Fan, X. Chen, J. Li, G. Shao, L. Qiu and Z. Ren, Eur. J. Med. Chem., 2013, 65, 21 CrossRef CAS PubMed.
- W. Huang, J. Li and L. Ou, Synth. Commun., 2007, 37, 2137 CrossRef CAS PubMed.
- (a) A. Kulkarni, M. Abid, B. Toeroek and X. Huang, Tetrahedron Lett., 2009, 50, 1791 CrossRef CAS PubMed; (b) G. Zhang, R. Cao, L. Guo, Q. Ma, W. Fan, X. Chen, J. Li, G. Shao, L. Qiu and Z. Ren, Eur. J. Med. Chem., 2013, 65, 21 CrossRef CAS PubMed; (c) Y. Song, J. Wang, S. F. Teng, D. Kesuma, Y. Deng, J. Duan, J. H. Wang, R. Z. Qi and M. M. Sim, Bioorg. Med. Chem. Lett., 2002, 12, 1129 CrossRef CAS.
- (a) W. Yin, P. V. V. SriramaSarma, J. Ma, D. Han, J. L. Chen and J. M. Cook, Tetrahedron Lett., 2005, 46, 6363 CrossRef CAS PubMed; (b) S. B. Dantale and B. C. G. Soderberg, Tetrahedron, 2003, 59, 5507 CrossRef CAS.
- (a) F. Gatta and D. J. Misiti, Heterocycl. Chem., 1987, 24, 1183 CrossRef CAS PubMed; (b) M. Cain, O. Campos, F. Guzman and J. M. Cook, J. Am. Chem. Soc., 1983, 105, 907 CrossRef CAS; (c) O. Campos, M. DiPierro, M. Cain, R. Mantei, A. Gawish and J. M. Cook, Heterocycles, 1980, 14, 975 CrossRef CAS.
- (a) S. Hibino, O. Miko, I. Masataka, S. Kohichi and I. Takashi, Heterocycles, 1985, 23, 261 CrossRef CAS; (b) D. Soerens, J. Sandrin, F. Ungemach, P. Mokry, G. S. Wu, E. Yamanaka, L. Hutchins, M. DiPierro and J. M. Cook, J. Org. Chem., 1979, 44, 535 CrossRef CAS; (c) R. T. Coutts, R. G. Micetich, G. B. Baker, A. Benderly, T. Dewhurst, T. W. Hall, A. R. Locock and J. Pyrozko, Heterocycles, 1984, 22, 131 CrossRef CAS.
- (a) I. J. W. Still and J. McNulty, Heterocycles, 1989, 29, 2057 CrossRef CAS; (b) M. Cain, R. W. Weber, F. Guzman, J. M. Cook, S. A. Barker, K. C. Rice, J. N. Crawley, S. M. Paul and P. Skolnick, J. Med. Chem., 1982, 25, 108 CrossRef; (c) W. Qifeng, C. Rihui, F. Manxiu, G. Xiangdong, M. Chunming, L. Jinbing, S. Huacan and P. Wenlie, Eur. J. Med. Chem., 2009, 44, 533 CrossRef PubMed.
- (a) W. O. Foye, X. Wang and W. Hongfu, Med. Chem. Res., 1997, 7, 180 CAS; (b) J. Kobayashi, J. Cheng, T. Ohta, S. Nozoe, Y. Ohizumi and T. Sasaki, J. Org. Chem., 1990, 55, 3666 CrossRef CAS; (c) C. Yeun-Mun and M. C. Hamann, Heterocycles, 2007, 71, 245 CrossRef.
- (a) H. R. Snyder, C. H. Hansch, L. Katz, S. M. Parmerter and E. C. Spaeth, J. Am. Chem. Soc., 1948, 70, 219 CrossRef CAS; (b) K. P. Lippke, W. G. Schunack, W. Wenning and W. E. Muller, J. Med. Chem., 1983, 26, 4993 CrossRef.
- (a) A. Meddour and J. Courtieu, Tetrahedron: Asymmetry, 2000, 11, 3635 CrossRef CAS; (b) M. Moreno-Manas, J. Bassa, N. Llado and R. Pleixats, J. Heterocycl. Chem., 1990, 27, 673 CrossRef CAS PubMed.
- (a) X. Yang, J. Wu, X. Mao, T. F. Jamison and T. A. Hatton, Chem. Commun., 2014, 50, 3245 RSC; (b) L. S. de Almeida, P. M. Esteves and M. C. S. de Mattos, Synlett, 2007, 1687 CAS.
- (a) M. Narender, M. S. Reddy and K. R. Rao, Synthesis, 2004, 1741 CAS; (b) K. Jeyakumar and D. K. Chand, Synthesis, 2009, 306 CAS.
- J. Prakash and S. Roy, J. Org. Chem., 2002, 67, 7861 CrossRef PubMed.
- C. Kuang, Q. Yang, H. Senboku and M. Tokuda, Synthesis, 2005, 1319 CrossRef CAS.
- (a) G. K. S. Prakash, T. Mathew, D. Hoole, P. M. Esteves, Q. Wang, G. Rasul and G. A. Olah, J. Am. Chem. Soc., 2004, 126, 15770 CrossRef CAS PubMed; (b) C. Y. Chen, T. Andreani and H. Li, Org. Lett., 2011, 13, 6300 CrossRef CAS PubMed.
- H. T. Clarke and L. D. Behr, Org. Synth., 1936, 16, 75 CrossRef CAS.
- Z. Yang and J. Xu, Synthesis, 2013, 45, 1675 CrossRef.
- (a) R. Ikeda, T. Kimura, T. Tsutsumi, S. Tamura, N. Sakai and T. Konakahara, Bioorg. Med. Chem. Lett., 2012, 22, 3506 CrossRef CAS PubMed; (b) J. G. Tang, H. Liu, Z. Y. Zhou and J. K. Liu, Synth. Commun., 2010, 40, 1411 CrossRef CAS PubMed; (c) R. Ikeda, T. Iwaki, T. Iida, T. Okabayashi, E. Nishi, M. Kurosawa, N. Sakai and T. Konakahara, Eur. J. Med. Chem., 2011, 46, 636 CrossRef CAS PubMed.
- Q. S. Guo and J. B. Bill, Tetrahedron Lett., 1994, 35, 1141 CrossRef.
- (a) T. Hino, Z. Lai, H. Seki, R. Hara, T. Kuramochi and M. Nakagawa, Chem. Pharm. Bull., 1989, 37, 2596 CrossRef CAS; (b) H. H. Wasserman and T. A. Kelly, Tetrahedron Lett., 1989, 30, 7117 CrossRef CAS.
- M. A. Ponce and R. Erra-Balsells, J. Heterocycl. Chem., 2001, 38, 1087 CrossRef CAS PubMed.
- J. B. Hudson, H. Saboune, Z. Abramowsgki, G. H. N. Towers and K. L. Rinehart Jr, Photochem. Photobiol., 1989, 50, 277 CrossRef PubMed.
- K. F. Kinzer and J. H. Cardellina II, Tetrahedron Lett., 1987, 28, 925 CrossRef CAS.
- (a) J. McNulty and I. W. J. Still, J. Chem. Soc., Perkin Trans. 1, 1994, 1329 RSC; (b) B. C. VanWagenen and J. H. Cardellina II, Tetrahedron Lett., 1989, 30, 3605 CrossRef CAS; (c) P. Rocca, F. Marsais, A. Godard and G. Queguiner, Synth. Commun., 1995, 25, 3373 CrossRef CAS PubMed.
- (a) J. D. Panarese and S. P. Waters, Org. Lett., 2010, 12, 4086 CrossRef CAS PubMed; (b) P. Molina, P. M. Fresneda and S. Garcia-Zafra, Tetrahedron Lett., 1995, 36, 3581 CrossRef CAS; (c) P. Rocca, F. Marsais, A. Godard, G. Queguiner, L. Adams and B. Alo, Tetrahedron Lett., 1995, 36, 7085 CrossRef CAS; (d) F. Nissen, V. Richard, C. Alayrac and B. Witulski, Chem. Commun., 2011, 47, 6656 RSC; (e) A. D. Yamaguchi, D. Mandal, J. Yamaguchi and K. Itami, Chem. Lett., 2011, 40, 555 CrossRef CAS; (f) C. M. Roggero, J. M. Giulietti and S. P. Mulcahy, Bioorg. Med. Chem. Lett., 2014, 24, 3549 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16221a |
| This journal is © The Royal Society of Chemistry 2015 |