Highly sensitive and selective detection of mercury ions based on up-conversion FRET from NaYF4:Yb3+/Er3+ nanophosphors to CdTe quantum dots†
Shaobo Cuiab,
Sai Xua,
Hongwei Song*a,
Wen Xua,
Xu Chena,
Donglei Zhoua,
Ze Yina and
Wei Han*b
aState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: songhw@jlu.edu.cn
bCollege of Physics, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: whan@jlu.edu.cn
First published on 13th November 2015
Abstract
The detection of Hg2+ has attracted considerable attention because of the serious health and environmental problems caused by it. There has been progress in the development of fluorescence biosensors based on quantum dots (QDs) for the detection of Hg2+. However, most of them are valid only in aqueous solution rather than in human serum due to the influence of protein autofluorescence in serum excited by ultraviolet or visible light. Herein, we designed and synthesized a novel NaYF4:Yb3+, Er3+ upconversion nanoparticle (UCNP)/CdTe QD composite probe for Hg2+ detection. The NaYF4:Yb3+, Er3+ UCNPs were synthesized via a solvothermal method. By grafting the CdTe QD probe onto the surface of the NaYF4:Yb3+, Er3+ UCNP, a fluorescence resonance energy transfer (FRET) biosensor for determination of Hg2+ ions was obtained under the pumping of 980 nm infrared light, which was capable of overcoming autoluminescence from serum. The spectral response towards Hg2+ suggested that the fluorescence intensity of the QDs reduced linearly with increasing Hg2+ concentration. The sensor showed high selectivity, a low detection limit of 15 nM and good linear Stern–Volmer characteristics, both in the buffer and serum. This biosensor has great potential for real applications of Hg2+ detection in biological and analytical fields.
1. Introduction
As a well-known health threat to human beings as well as many animal species, mercury and its complexes are considered as unpopular materials due to their participation in many biological processes.1,2 Many vital organs such as the human heart, kidney, stomach and intestines can be poisoned and damaged when the Hg2+ concentration reaches a level of 25 nM.3 The excess Hg2+ in human body can initiate a series of diseases including nosebleed, headache, nerve disorder, perforation of stomach, intestines septum and even acute renal failure.4 The increasing Hg2+ contamination in our living environment and ecosystem has created significant concerns. From the viewpoint of environment protection and health concerns, developing an effective method for the sensitive detection of trace amount of Hg2+ is very important and has attracted a significant amount of attention.5A variety of traditional methods including atomic absorption spectrometry, atomic fluorescence spectrometry, inductively coupled plasma mass spectroscopy, X-ray absorption spectrometry and electrochemical method have been widely used for mercury ions detection.6–9 However, these methods require expensive instruments and complicated sample preparation process. In order to overcome these drawbacks, it is highly desirable to develop facile, economical and rapid methodologies with favorable sensitivity and selectivity for mercury detection. Relative to the above methods, the fluorescence method has the advantages of higher sensitivity, simplicity and lower cost for the monitoring of metal ions, which translates molecular recognition information into tangible fluorescence signals. In the past years, fluorescent probes for Hg2+ was usually based on organic dyes, noble metal ions and DNA, via Hg2+-induced changes in fluorescence, has abstracted much attention and become a very active field of research.10,11 Although progress in developing these methods has occurred, problems remain in applying them to biomonitoring. For instance, organic luminescence dyes are photo-bleached after continuous irradiation by ultraviolet or visible light, and the expensive price of noble metal ions lead to the rise cost of preparation and experiment. Recently, quantum dots (QDs) have become a promising fluorescence probes for mercury detection with the advantage of high sensitivity, simplicity and low cost because of the attractive optical properties, including tunable emission spectra, excellent photochemical stability. For instance, Ding et al. prepared cysteamine capped CdTe QDs to detect Hg2+. The results show the detection of Hg2+ based QDs exhibited excellent sensitivity due to the specific and strong affinity of ligands capped on the QDs with Hg2+ and the unique luminescence properties of QDs.12 However, this method is valid in aqueous solution rather than in human serum. The porphyrin and carotenoids in the serum can be excited by visible light and emit fluorescence ranging of 500–600 nm, thus influence detection of Hg2+.13
On the other hand, rare earth (RE)-activated UCNPs can convert infrared light to visible through two-photon or multi-photon processes via intermediate states.14 This unique mechanism allows them to display some special advantages in bio-application, such as a large anti-stokes shift of several hundred nanometers, drastically improving signal-to-noise ratio and reducing background signals owing to the autofluorescence of the other biofluorescent components not being excited with NIR radiation,15 remarkable light penetration depth in tissue,16 less harm to cells and tissues in vitro or in vivo,17 and no photobleaching.18,19 Many investigation using UCNPs as bio-imaging or bio-detection have been reported due to these advantages.20–25 However, work related to the detection of Hg2+ using NaYF4:Yb3+, Er3+ UCNPs/CdTe QDs nanocomposite as Hg2+ sensor has not been reported yet. It is expected that the Hg2+ probe based on NaYF4:Yb3+, Er3+ UCNPs/CdTe QDs nanocomposite can avoid background florescence in the serum.
Guided by above considerations, in this paper, we devised and prepared NaYF4:Yb3+, Er3+ UCNPs/CdTe QDs nanocomposite as Hg2+ sensor. The hybrid can maintain the sensitivity of CdTe QDs to Hg2+, and through the FRET from NaYF4:Yb3+, Er3+ UCNPs to CdTe QDs, it is promised to detect Hg2+ under the excitation of infrared light, which can avoid background florescence of serum. The principle of upconversion FRET mercury-ion detection by employing UCNPs as energy donors and QDs as an energy acceptor is illustrated in Scheme 1. The central wavelength of the absorption band of the QDs locates at 547 nm, which mainly overlaps with the 546 nm emission of NaYF4:Yb3+, Er3+ UCNPs. As CdTe QDs was electrostatically absorbed on the surface of the NaYF4:Yb3+, Er3+ UCNPs, an efficient FRET from UCNPs to QDs was realized when UCNPs was excited by 980 nm light. Because of their small size and high surface area-to-volume ratio, the photoluminescence of QDs is very sensitive to the surface states. After adding the Hg2+ ions, the surface states of QDs changed due to the specific interaction between Hg2+ and carboxyl on the surface of QDs, leading to fluorescence quenching of QDs. Ultimately, the Hg2+ ions were detected quantitatively through the spectrum change of QDs.
 | ||
| Scheme 1 Principle of upconversion Hg2+ ions FRET detection by employing UCNPs as donor and QDs as acceptor. | ||
2. Experimental section
2.1. Synthesis of PEI-modified NaYF4:Yb3+, Er3+ UCNPs
PEI-modified water-soluble NaYF4:Yb3+, Er3+ UCNPs were synthesized via a solvothermal method following a procedure reported by Liu et al.26 Briefly, NaCl (2.5 mmol), PEI (0.4 g), Y(NO3) (0.77 mmol), Yb(NO3) (0.2 mmol), and Er(NO3) (0.03 mmol) were dissolved in ethylene glycol (15 mL) under vigorous stirring. After the solution became transparent, NH4F (4 mmol) in ethylene glycol (10 mL) was dropwise added to the solution under vigorous stirring. After stirring for another 10 min, the entire mixture solution was transferred into a 25 mL Teflon-lined stainless steel autoclave. The autoclave was sealed and heated under 200 °C for 2 h. After the autoclave was cooled down to room temperature naturally, NaYF4:Yb3+, Er3+ UCNPs were collected by centrifugation and washed with deionized water and alcohol three times.2.2. Preparation of water-soluble CdTe QDs
Stable water-solution CdTe QDs were prepared as described in previous papers.27 Briefly, cadmium chloride (CdCl2·2.5H2O, 0.04 M, 16 mL) was diluted to 200 mL in a one-necked flask, and trisodium citrate dehydrate (400 mg), Na2TeO3 (0.01 M, 4 mL), MSA (200 mg), and sodium borohydride (NaBH4, 400 mg) were added with stirring. When the color of the solution changed to green, the flask was attached to a condenser and refluxed under open-air conditions for 6 h. The resulting CdTe QDs were washed with ethanol and separated by centrifugation. Finally, the prepared CdTe QDs were dispersed in water.2.3. Detection of FRET process from UCNPs to QDs
A specified concentration of UCNPs was transferred to a cuvette, and then different concentration of QDs was added to the solution. After 10 min incubation at room temperature, the luminescence spectra were measured with a photomultiplier combined with a monochromator for signal collection from 350 nm to 750 nm. A continuous 980 nm diode laser was used to pump the samples to investigate steady-state spectra. In the measurements of luminescent dynamics, the samples were pumped by a laser system consisting of a Nd:YAG pumping laser (1064 nm), the third-order harmonic generator (355 nm) and a tunable optical parameter oscillator (OPO, Continuum Precision II 8000). Pulse duration was 10 ns, repetition frequency 10 Hz, and line width 4–7 cm−1.2.4. Sensitivity and selectivity of Hg2+ detection in PBS buffer
HgSO4 was used for the Hg2+-sensitivity studies. UCNPs/CdTe QDs nanocomposites were mixed in the buffer after 10 min incubation at room temperature. Different concentrations of Hg2+ were added to the mixture solution, and then emission spectra were measured with the same equipment described in section 2.3. To determine FRET probe selectivity, various ions, including Mg2+, K+, Na+, Cl−, Zn2+, Cu2+, Cd2+, Ag+, Al3+ and Ni2+ were prepared at a concentration of 0.5 mM. The solutions above and 1 μM of Hg2+ solution were added to the mixture individually and changes in fluorescence were monitored.In the control experiment, the QD solution was transferred to a cuvette, and then a specific concentration of Hg2+ was added. The emission spectra were measured under 365 nm excitation with a SENS-9000 spectrometer.
2.5. Detection of Hg2+ in real samples
The UCNPs/CdTe QDs nanocomposites were incubated in the serum (0.1 g mL−1). After 10 min incubation at room temperature, different concentration of Hg2+ was added to the mixture solution. The emission spectra were measured with the same equipment as the detection process in the buffer.In the control experiment, QDs were mixed in the serum, and then different concentration of Hg2+ was added; the emission spectra were measured with the same equipment as the detection process in the buffer.
3. Characterization
Transmission electron micrographs were obtained with a Hitachi H-800 transmission electron microscope (TEM) operating at an acceleration voltage of 200 kV. High-resolution transmission electron microscope (HR-TEM) images and elemental mapping were recorded on a FEI Tecnai G2 S-Twin microscope operated with an acceleration voltage of 200 kV. The phase structure of the samples was characterized by X-ray diffraction (XRD) (RigakuD/max-rA power diffractometer using Cu-KR radiation (λ = 1.54178 Å). Ultraviolet-visible (UV-vis) absorption spectra were measured with a Shimadzu UV-3101PC UV-vis scanning spectrophotometer ranging of 200–1100 nm. The emission spectra were recorded at room temperature using a HitachiF-4500 spectrophotometer. The luminescence dynamics were studied using a laser system consisting of a Nd:YAG pumping laser (1064 nm), a third-order harmonic generator (355 nm), and a tunable optical parameter oscillator (OPO, Continuum Precision II 8000). Pulse duration was 10 ns, repetition frequency 10 Hz, and line width 4–7 cm−1.4. Results and discussion
4.1. Morphology and structure of NaYF4:Yb, Er UCNPs and CdTe QDs
The UCNPs and QDs could be well-dispersed in water to form stable colloidal solutions directly without further surface modification. Fig. 1(a) exhibits XRD patterns of the NaYF4:Yb, Er UCNPs and CdTe QDs. From the XRD patterns it can be observed that all the diffraction peaks are indexed to the cubic phase structure of NaYF4 crystals, which is in accordance with JCPDS card no. 77-2042, suggesting high product crystallinity. The mean crystallite size of the product was estimated from the XRD pattern according to the Scherrer formula D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, where λ is the X-ray wavelength (0.15406 nm), β is the full-width at half-maximum, θ is the diffraction angle, and K is a constant (0.89).28 The estimated mean crystallite size of NaYF4 crystals is 16.9 nm. It can be seen from the XRD patterns of the CdTe QDs that all the diffraction peaks can be indexed in accordance with the cubic phase CdTe QDs (JCPDS: 65-1047), indicating that the prepared sample is pure in phase. Fig. 1(b) shows the TEM image of spherical morphology of the NaYF4 UCNPs. It is with an average diameter of 17.1 nm, which is well accordance with the theoretical calculation. Fig. 1(c) shows the HR-TEM picture of the water-dispersed CdTe QDs, in which the average size of pristine CdTe QDs was estimated to be 3.5 nm. Fig. 1(d) demonstrates the HR-TEM image of the NaYF4:Yb3+, Er3+/CdTe QDs composites, CdTe QDs and NaYF4:Yb3+, Er3+ UCNPs. It can be seen that the CdTe QDs were adsorbed on the surface of NaYF4 UNCPs. The lattice spacing of the QDs was 0.3178 nm, corresponding to the (002) planes in the cubic phase of CdTe QDs, and the lattice spacing of the NaYF4 UCNPs was 0.2735 nm, corresponding to the (200) planes in the cubic phase of NaYF4 UCNPs. Fig. 1(e) shows the zeta potential between the NaYF4 UNCPs, CdTe QDs and composite. It can be seen that NaYF4 UNCPs have positive charge (ζ potential = +35.3 mV), CdTe QDs have negative (ζ potential = −12.34 mV) and the composite have negative charge (ζ potential = −3.5 mV). The opposite potential contributes to strong electrostatic interaction between UCNPs and QDs. Fig. S2† displays the TEM image and elemental mapping of NaYF4/CdTe QDs. The EDX analysis indicates that the CdTe QDs were successfully absorbed on the surface of NaYF4 UCNPs, and the concentration of QDs is determined to be 32%, which is a little bit smaller than its starting concentration (45%). The formation of the CdTe-surrounded NaYF4 structure is attributed to not only the electrostatic interaction between UCNPs and QDs, but also the large difference in size of CdTe QDs and NaYF4 UCNPs.29 The size of CdTe QDs is much smaller than that of NaYF4, leading to CdTe QDs adsorbed on NaYF4 rather than in reverse. As mentioned above, it can be confirmed that the CdTe QDs were successfully absorbed on the surface of NaYF4 UCNPs.
θ, where λ is the X-ray wavelength (0.15406 nm), β is the full-width at half-maximum, θ is the diffraction angle, and K is a constant (0.89).28 The estimated mean crystallite size of NaYF4 crystals is 16.9 nm. It can be seen from the XRD patterns of the CdTe QDs that all the diffraction peaks can be indexed in accordance with the cubic phase CdTe QDs (JCPDS: 65-1047), indicating that the prepared sample is pure in phase. Fig. 1(b) shows the TEM image of spherical morphology of the NaYF4 UCNPs. It is with an average diameter of 17.1 nm, which is well accordance with the theoretical calculation. Fig. 1(c) shows the HR-TEM picture of the water-dispersed CdTe QDs, in which the average size of pristine CdTe QDs was estimated to be 3.5 nm. Fig. 1(d) demonstrates the HR-TEM image of the NaYF4:Yb3+, Er3+/CdTe QDs composites, CdTe QDs and NaYF4:Yb3+, Er3+ UCNPs. It can be seen that the CdTe QDs were adsorbed on the surface of NaYF4 UNCPs. The lattice spacing of the QDs was 0.3178 nm, corresponding to the (002) planes in the cubic phase of CdTe QDs, and the lattice spacing of the NaYF4 UCNPs was 0.2735 nm, corresponding to the (200) planes in the cubic phase of NaYF4 UCNPs. Fig. 1(e) shows the zeta potential between the NaYF4 UNCPs, CdTe QDs and composite. It can be seen that NaYF4 UNCPs have positive charge (ζ potential = +35.3 mV), CdTe QDs have negative (ζ potential = −12.34 mV) and the composite have negative charge (ζ potential = −3.5 mV). The opposite potential contributes to strong electrostatic interaction between UCNPs and QDs. Fig. S2† displays the TEM image and elemental mapping of NaYF4/CdTe QDs. The EDX analysis indicates that the CdTe QDs were successfully absorbed on the surface of NaYF4 UCNPs, and the concentration of QDs is determined to be 32%, which is a little bit smaller than its starting concentration (45%). The formation of the CdTe-surrounded NaYF4 structure is attributed to not only the electrostatic interaction between UCNPs and QDs, but also the large difference in size of CdTe QDs and NaYF4 UCNPs.29 The size of CdTe QDs is much smaller than that of NaYF4, leading to CdTe QDs adsorbed on NaYF4 rather than in reverse. As mentioned above, it can be confirmed that the CdTe QDs were successfully absorbed on the surface of NaYF4 UCNPs.
4.2. FRET process from UCNPs to QDs
FRET is a non-radiation process characterized by energy transfer between an excited donor and an acceptor through long-range dipole–dipole interaction, which is widely used as a spectroscopic ruler in biosensing and bioimaging.25 An efficient FRET process requires the following: (1) the acceptor absorption spectrum overlaps with the donor emission spectrum; and (2) the distance between a donor and an acceptor must be linked in close proximity, typically less than a few nanometers.30 Fig. 2(a) shows the upconversion emission spectrum of NaYF4:Yb3+, Er3+ nanoparticles under 980 nm excitation, absorption and emission spectra of the CdTe QD1. In the emissions of the Er3+ ions, the 2H9/2, 2H11/2, 4S3/2, and 4F9/2–4I15/2 transitions were observed, locating at 412, 526, 546, and 658 nm, respectively. The central wavelength of the absorption band of the QD1 locates at 547 nm, which exactly overlaps with the 546 nm (4S3/2–4I15/2) emission of NaYF4:Yb3+, Er3+ UCNPs. The emission band of the QD1 locates at 580 nm, which corresponds to an orange color, does not overlap with the emission of Er3+ ions. The QDs emission originates from the radiation recombination of electrons and holes trapped in defect states.31 There are sufficient conditions for UCNPs-QDs non-radiative energy transfer (NRET) to take place because the green luminescence of the donor (Er3+) overlaps with the strong absorption band of the acceptor (CdTe). Another important reason that CdTe was selected as the FRET acceptor is that the UCNPs can be excited using an NIR laser at 980 nm where CdTe cannot be photo-excited, thereby avoiding CdTe excitation by external light and eliminating luminescent background interference.The emission spectra of Er3+ ions versus the concentration of the QD1 in the NaYF4:Yb3+, Er3+/CdTe QD1 mixture solution under the excitation of 980 nm laser diode are shown in Fig. 2(b), the emission from 4F9/2–4I15/2 transitions around 658 nm situates relatively far away from the absorption and emission spectra of the CdTe QD1 and is almost unchanged, so it is normalized for comparison. It can be seen that the emission band of the QD1 gradually increases with the addition of QD1, while the green emission at 530–570 nm of Er3+ gradually decreases, indicating the appearance of FRET from Er3+ to CdTe QD1. The blue and red emission at 400–430 and 635–670 nm are almost unchanged.
To further confirm the FRET process, the decay dynamics for 4S3/2–4I15/2 and 4F9/2–4I15/2 transition processes of Er3+ with different QD1 concentration were investigated. All the dynamic curves exhibited exponential decay (as shown in Fig. S3 and S4†). According to the inverse of 4S3/2–4I15/2 and 4F9/2–4I15/2 transition decay time constant as shown in Fig. 2(c), it can be clearly seen that with increasing concentration of QD1 from 0 to 45%, the decay rate for 4S3/2–4I15/2 transition of Er3+ linearly increases from 0.56 μs−1 to 0.78 μs−1, which is evidence for NRET from 4S3/2 of Er3+ to the QD1. Meanwhile, the decay time for 4F9/2–4I15/2 transition of Er3+ remains almost unchanged with concentration variation, which means that there is no energy transfer from 4F9/2 of Er3+ to QD1. The energy transfer efficiency from 4S3/2 of Er3+ to QD1 can be calculated as follows:15
| η = 1 − τD1/τD |
As a contrast, CdTe QD2 with the central absorption band at 465 nm was synthesized. From Fig. 3(a) it can be seen that the central wavelength of the absorption band for QD2 does not overlap with any emission of NaYF4:Yb3+, Er3+ UCNPs. The emission spectra of Er3+ ions versus the concentration of the QD2 in the NaYF4:Yb3+, Er3+/CdTe QD2 mixture solution under the excitation at 980 nm was shown in Fig. 3(b). While exciting Er3+ ions to excited states, no emission from CdTe was identified, implying FRET did not happen. The decay dynamics for 4S3/2–4I15/2 and 4F9/2–4I15/2 transition processes of Er3+ with different QD1 concentration were investigated. All the dynamic curves exhibited exponential decay (as shown in Fig. S6 and S7†), the decay rates of 4S3/2–4I15/2 and 4F9/2–4I15/2 of Er3+ transition on concentration of QD2 were shown in Fig. S8.† The results indicate the decay rates for 4S3/2–4I15/2 and 4F9/2–4I15/2 of Er3+ almost unchanged. This further means that there is no energy transfer from NaYF4:Yb3+, Er3+ to QD2.
The schematic for the upconversion luminescence and energy transfer processes of NaYF4:Yb3+, Er3+/CdTe QDs composites is illustrated in Fig. 4; as shown in Fig. 4, under the pumping of a 980 nm light, the excited states of Er3+ ions (2H11/2, 4S3/2) as well as 4F9/2 are populated through two-step energy transfer processes from Yb3+ to Er3+ ions. The transitions of 2H11/2, 4S3/2–4I15/2 match well with the energy gap between the conduction band (CB) and valance band (VB) of the CdTe QDs, resulting in the NRET from UCNPs to QDs.
4.3. Fluorescence response characteristics of FRET probe for Hg2+ detection
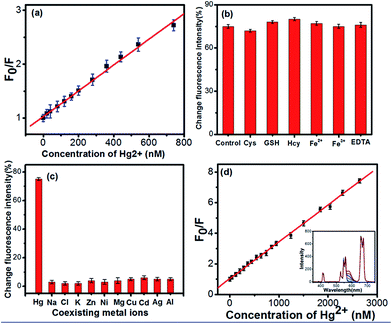
In the Hg2+ sensing system, we chose UCNPs as the energy donors, which can drastically improve signal-to-noise ratio and reduce background signals owing to the autofluorescence of the other biofluorescent components not being excited with NIR radiation, to improve the linearity and accuracy of detection. With the addition of CdTe QDs to the UCNPs, excitation of NaYF4:Yb3+, Er3+ UCNPs triggers energy transfer from Er3+ to CdTe QDs within a given proximity through electrostatic interaction, and results in emission from QDs at 580 nm, and the weakening of 2H11/2, 4S3/2–4I15/2 transitions for Er3+. The carboxyl capping layer was crucial for CdTe QD luminescent efficiency and water stability. Thus, as adding the Hg2+ ions, the surface states of QDs changed with the addition of Hg2+, leading to fluorescence quenching of QDs.To evaluate the sensitivity of this method, the fluorescence intensities of PBS buffer containing UCNPs, QDs and different amounts of Hg2+ were monitored. The extent of fluorescence quenching of QDs was dependent on the concentration of Hg2+, as shown in Fig. 5(a). The fluorescence quenching is best described by the Stern–Volmer equation:32
| F0/F = 1 + K[Q], |
Because interference from glutathione (GSH), cysteine (Cys) and homocysteine (Hcy) in human serum may influence Hg2+ detection, the anti-interference performance of the biosensor was measured. Fig. 5(b) shows the response of 1 μM Hg2+ in the absence (control) and presence of 1 mM GSH, Cys, Hcy, Fe2+/Fe3+, and EDTA in the buffer, respectively. Adding each material did not significantly influence Hg2+ detection, which illustrates the biosensor's excellent anti-interference performance.
To estimate the selectivity of the FRET probe for Hg2+ detection, Mg2+, K+, Na+, Cl−, Zn2+, Cu2+, Cd2+, Ag+, Al3+ and Ni2+ were investigated along with Hg2+. As shown in Fig. 5(c), only Hg2+ led to a dramatic change of fluorescence intensity, and no significant fluorescence intensity variation was found with the presence of other ions. Interfering ions exhibited low selectivity coefficients in the range of 0.024–0.159. These results clearly demonstrate the great selectivity of the biosensor for the target ion.
Because of superior sensitivity, selectivity, and anti-interference performance of our FRET probe in the buffer solution, we explored its capability to detect Hg2+ in human serum (0.1 g mL−1). As shown in the Fig. 5(d) inset, upon addition of Hg2+ ions, the emission from 570 to 630 nm of QDs decreased continuously in intensity, but without a peak shift. Meanwhile, the emission intensity of Er3+ in the range of 400–570 nm and 640–720 nm did not change. The decay dynamics for 4S3/2–4I15/2 transition processes of Er3+ with different Hg2+ concentration were further investigated. As shown in Fig. S9,† all the dynamic curves exhibited exponential decay process, it can be seen that the decay rates for 4S3/2–4I15/2 of Er3+ almost unchanged. The results indicate that Hg2+ should not affect the luminescence of Er3+ and the ET process from Er3+ to QDs, instead, affect the luminescence of QDs. The carboxyl capping layer was crucial for CdTe QD luminescent efficiency and water stability. Thus, as adding the Hg2+ ions, the surface states of QDs changed with the addition of Hg2+, leading to fluorescence quenching of QDs. The calibration plot of F0/F versus Hg2+ concentration in human serum (0.1 g mL−1) showed a good linear relationship in the 10–2800 nmol L−1 range, as shown in Fig. 5(d). The plots are well described by the Stern–Volmer equation (R = 0.993), and K was determined to be 2.57 μM−1. Fluorescence intensity ratio of FRET sensor as a function of Hg2+ concentration detected in human serum (0.15 g mL−1) was further measured, as shown in Fig. S10.† It can be seen that the calibration plot of F0/F versus Hg2+ concentration in human serum (0.15 g mL−1) showed a good linear relationship in the 10–2800 nmol L−1 range. The plots are well described by the Stern–Volmer equation (R = 0.994), and K was determined to be 2.60 μM−1. The results were similar to those detected in the PBS buffer. The results indicate that our FRET probe has superior sensitivity in different concentration of human serum. The theoretical detection limit was evaluated using 3σ/S and determined to be 15 nM in human serum of 0.1 g mL−1 and 17 nM in human serum of 0.15 g mL−1, where σ represents the standard deviation of the blank signal. The σ can be calculated as13
Actually, CdTe QDs probes can be used directly for the detection of Hg2+ in aqueous solution. In order to confirm that the present NaYF4:Yb3+, Er3+ UCNPs/CdTe QDs hybrid probes was superior to the single QD probes, only CdTe QDs probes were used for Hg2+ detection, in buffer and human serum, respectively. Fig. 6(a) shows the calibration plot of F0/F versus Hg2+ concentration for the CdTe QDs probes under 365 nm excitation. It can be seen that in the PBS solution, the calibration plot of F0/F versus Hg2+ concentration show a good linear relationship in the range of 20–2800 nM, indicating that CdTe QDs also exhibit good performance of Hg2+ detection in the PBS buffer, the fluorescence spectra of QDs with addition of various Hg2+ concentrations was shown in Fig. S11.† Unfortunately, the calibration plot of F0/F versus Hg2+ concentration shows no linearity from 20 to 2800 nM in the serum. In order to clarify its origin, the emission spectra of the mixture of CdTe QDs and serum under the excitation of 365 nm were measured, as shown in Fig. 6(b). It can be seen that the emission band of the CdTe QDs locates at 590 nm, after the serum was added to the CdTe QDs, the emission spectra of the mixture of QDs and serum under the excitation of 365 nm was measured, actually, the emission band of the QDs still locates at 590 nm, meanwhile, the emission band of the serum locates at 520 nm under 365 nm excitation, leading to the emission spectra of the mixture of QDs and serum under the excitation of 365 nm blue-shifted from 590 nm to 555 nm comparing to the QDs emission, attributed to the spectra superposition of QDs and serum. After Hg2+ ions was added to the mixture of QDs and serum, the emission intensity of QDs weaken with the increase of Hg2+ ions concentration, while the emission intensity of serum remained virtually unaltered, leading to the fluorescence intensity ratio of the mixture of QDs and serum versus Hg2+ concentration shows no linearity from 20 to 2800 nM in human serum. This shows that the fluorescence of serum had great impact on the fluorescence of the mixture of CdTe QDs and serum, leading to no linearity in the serum. The serum emission is caused mainly by porphyrin, serum proteins, and carotenoids, as reported previously.33
Compared to five other reported methods based on different fluorescence probes, the proposed method shows better linear range and sensitivity as shown in Table 1. Liu et al. reported a sensor synthesized with rhodamine derivative covalently liked onto graphene quantum dots for Hg2+ detection in Hela cells, the sensor was cell permeable and could be used for monitoring Hg2+ in living cells, however, the best linear response concentration range of Hg2+ in the sensor was from 0.6 to 12 μM with a correlation coefficient of 0.992, and the detection limit was estimated to be 0.23 μM.39 It can be concluded that the system we established is a comparatively facile method to detect Hg2+ ions in human serum.
| Fluorescence probes | Linear range (μM) | Detect limit (μM) | References |
|---|---|---|---|
| UCNPs/QDs | 0.01–2.8 | 0.015 | This work |
| Cysteine functionalized silver nanoparticles | 0–55 | 0.065 | 34 |
| DNA-functionalized gold nanoparticles | 0.05–2.5 | 0.025 | 35 |
| Carbon dots | 0–2.69 | 1.3 | 36 |
| Functionalized carbon dots | 0.1–2.69 | 2.69 | 37 |
| Polymer sensor | 1–30 | 0.728 | 38 |
5. Conclusions
In conclusion, we developed a new type of mercury biosensor based on FRET from NaYF4:Yb3+, Er3+ UCNPs to the CdTe QDs, which overcomes the lack of NIR-excitable probes for mercury ions. The sensor can be used for mercury-ion sensing both in the PBS buffer and in serum with comparable performance, proving that the biosensor is capable of overcoming autofluorescence from serum excitation due to visible light. When applied to detect mercury ions in human serum, the sensor had a good linear relationship (R = 0.996), and a low detection limit (15 nM), thereby easily detecting the 25 nM safety limit of mercury in the blood. Thus, the sensor we designed has great potential for real applications in mercury detection in biological and analytical fields.Acknowledgements
This work was supported by the Major State Basic Research Development Program of China (973 Program) (no. 2014CB643506), the National Natural Science Foundation of China (Grant no. 11374127, 11304118, 61204015, 81201738, 81301289, 61177042, and 11174111), the program of Chang Jiang Scholars and Innovative Research Team in University (no. IRT13018), the Graduate Innovation Fund of Jilin University (no. 2015064).Notes and references
- T. W. Clarkson, L. Magos and G. J. Myers, N. Engl. J. Med., 2003, 349, 1731–1737 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- T. W. Clarkson and L. Magos, Crit. Rev. Toxicol., 2006, 36, 609–662 CrossRef CAS PubMed.
- D. S. Zou, D. S. Zhai, H. Y. Sun and K. Zhang, Spectrochim. Acta, Part A, 2014, 118, 1062–1067 CrossRef CAS PubMed.
- D. Sánchez-Rodas, W. T. Corns, B. Chen and P. B. Stockwell, J. Anal. At. Spectrom., 2010, 25, 933–946 RSC.
- K. Leopold, M. Foulkes and P. J. Worsfold, Anal. Chim. Acta, 2010, 663, 127–138 CrossRef CAS PubMed.
- J. Y. Pei, H. Zhu, X. L. Wang, H. C. Zhang and X. R. Yang, Anal. Chim. Acta, 2012, 757, 63–68 CrossRef CAS PubMed.
- Q. Mu, Y. Li, H. Xu, Y. F. Ma, W. H. Zhu and X. H. Zhong, Talanta, 2014, 119, 564–571 CrossRef CAS PubMed.
- S. J. Liu, Z. J. Shi, W. J. Xu, H. R. Yang, N. Xi, X. M. Liu, Q. Zhao and W. Huang, Dyes Pigm., 2014, 103, 145–153 CrossRef CAS.
- W. J. Yan, Y. L. Wang, H. Zhuang and J. H. Zhang, Biosens. Bioelectron., 2015, 68, 516–520 CrossRef CAS PubMed.
- X. J. Ding, L. B. Qu, R. Yang, Y. C. Zhou and J. J. Li, Luminescence, 2015, 30, 465–471 CrossRef CAS PubMed.
- S. Xu, S. H. Xu, Y. S. Zhu, W. Xu, P. W. Zhou, C. Y. Zhou, B. Dong and H. W. Song, Nanoscale, 2014, 6, 12573–12579 RSC.
- J. H. Zhang, Z. D. Hao, J. Li, X. Zhang, Y. S. Luo and G. H. Pan, Light: Sci. Appl., 2015, 4, e239 CrossRef CAS.
- A. Bednarkiewicz, M. Nyk, M. Samoc and W. Strek, J. Phys. Chem. C, 2010, 114, 17535–17541 CAS.
- L. Q. Xiong, Z. G. Chen, Q. W. Tian, T. Y. Cao, C. J. Xu and F. Y. Li, Anal. Chem., 2009, 81, 8687–8694 CrossRef CAS PubMed.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834 CrossRef CAS PubMed.
- M. X. Yu, F. Y. Li, Z. G. Chen, H. Hu, C. Zhan, H. Yang and C. H. Huang, Anal. Chem., 2009, 81, 930–935 CrossRef CAS PubMed.
- Y. Park, J. H. Kim, K. T. Lee, K. S. Jeon, H. B. Na, J. H. Yu, H. M. Kim, N. Lee, S. H. Choi and S. B. Aik, Adv. Mater., 2009, 21, 4467–4471 CrossRef CAS.
- L. H. Fischer, G. S. Harms and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 4546–4551 CrossRef CAS PubMed.
- D. E. Achatz, R. J. Meier, L. H. Fischer and O. S. Wolfbeis, Angew. Chem., Int. Ed., 2011, 50, 260–263 CrossRef CAS PubMed.
- H. S. Mader and O. S. Wolfbeis, Anal. Chem., 2010, 82, 5002–5004 CrossRef CAS PubMed.
- Y. Liu, M. Chen, T. Y. Cao, Y. Sun, C. Y. Li, Q. Liu, T. Yang, L. M. Yao, W. Feng and F. Y. Li, J. Am. Chem. Soc., 2013, 135, 9869 CrossRef CAS PubMed.
- S. J. Wu, N. Duan, Z. Shi, C. C. Fang and Z. P. Wang, Talanta, 2014, 128, 327–336 CrossRef CAS PubMed.
- Q. Liu, J. J. Peng, L. N. Sun and F. Y. Li, ACS Nano, 2011, 5, 8040–8048 CrossRef CAS PubMed.
- F. Wang and X. G. Liu, J. Am. Chem. Soc., 2008, 130, 5642 CrossRef CAS PubMed.
- L. H. Jin, L. Shang, J. F. Zhai, J. Li and S. J. Dong, J. Phys. Chem. C, 2010, 114, 803–807 CAS.
- S. B. Cui, Y. S. Zhu, W. Xu, P. W. Zhou, L. Xia, X. Chen, H. W. Song and W. Han, Dalton Trans., 2014, 43, 13293–13298 RSC.
- T. T. Li, Z. N. Wu, T. T. Huang, J. l. Liu, L. Rong, S. J. Lan, Z. X. Guo, H. Zhang and B. Yang, RSC Adv., 2015, 5, 48024–48030 RSC.
- R. Gill, M. Zayats and I. Willner, Angew. Chem., Int. Ed., 2008, 47, 7602 CrossRef CAS PubMed.
- Y. Kobayashi, L. Y. Pan and N. Tamai, J. Phys. Chem. C, 2009, 113, 11783 CAS.
- E. M. Ali, Y. G. Zheng, H. H. Yu and J. Y. Ying, Anal. Chem., 2007, 79, 9452 CrossRef CAS PubMed.
- J. Yu, J. W. Meng, Y. Li, J. Ma and R. E. Rong, Spectrosc. Spectral Anal., 2004, 24, 981 CAS.
- L. Shang, J. Yin, J. Li, L. Jin and S. Dong, Biosens. Bioelectron., 2009, 25, 269–274 CrossRef CAS PubMed.
- C. W. Liu, C. C. Huang and H. T. Chang, Langmuir, 2008, 24, 8346–8350 CrossRef CAS PubMed.
- H. M. R. Goncalves, A. J. Duarte and J. C. G. E. daSilva, Biosens. Bioelectron., 2010, 26, 1302–1306 CrossRef CAS.
- H. Goncalves, P. A. S. Jorge, J. R. A. Fernandes and J. C. G. E. daSilva, Sens. Actuators, B, 2010, 145, 702–707 CrossRef CAS.
- J. Li, Y. Wu, F. Song, G. Wei, Y. Cheng and C. Zhu, J. Mater. Chem., 2012, 22, 478–482 RSC.
- M. P. Liu, T. Liu, Y. Li, H. Xu, B. Z. Zheng, D. M. Wang, J. Du and D. Xiao, Talanta, 2015, 143, 442–449 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra16200a |
| This journal is © The Royal Society of Chemistry 2015 |