Biocomposites for wound-healing based on sol–gel magnetite
Abstract
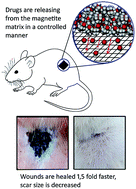
Currently, efficient wound-healing materials are booming due to increasing health care costs and world population aging, but also because of a sharp increase in the incidence of diabetes and obesity. Exacting demands are placed upon modern wound-healing materials as these should affect all stages of healing by accelerating them. In this paper, we demonstrate for the first time that drug entrapped magnetite xerogels can be effectively used for this purpose. To prepare a healing biocomposite, we have combined four medicaments in a magnetite matrix: chlorhexidine digluconate as an antimicrobial agent, lidocaine as a painkiller, prednisolone as an anti-inflammatory agent and chymotrypsin as a necrolytic agent. Compared to the control group, the wound healing rate with a biocomposite exhibited a ∼1.5-fold increase (21 and 14 days for complete healing, respectively). Moreover application of a magnetite-based biocomposite provided strong scar size decrease. Characteristics of the magnetite matrix as well as wound-healing composite material are fully described by XRD, XPS, SEM, TEM and N2 physisorption analysis.

 Please wait while we load your content...
Please wait while we load your content...