Polyaniline and iron based catalysts as air cathodes for enhanced oxygen reduction in microbial fuel cells
Abstract
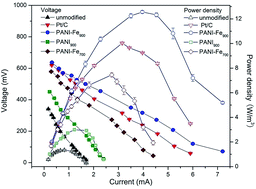
The catalyst for oxygen reduction in a cathode is vital for power production in microbial fuel cells (MFCs). In this study, non-precious metal catalysts were prepared by a high-temperature treatment of the iron containing polyaniline as both nitrogen and carbon precursor. These catalysts showed very positive onset potentials and less than 3% yield of hydrogen peroxide in the whole potential range, which matched the state-of-the-art Pt/C. The MFC with a bare cathode only produced a maximum power density of 1.32 W m−3, while the MFCs with a PANI900, PANI–Fe700, and PANI–Fe900 cathode had a maximum power density of 3.00 W m−3, 7.45 W m−3, and 12.54 W m−3, respectively. Physical and chemical characterizations of the catalysts indicated that iron coordinated with pyridinic nitrogen hosted in micropores was responsible for the high catalytic activity. These results demonstrate that these catalysts are excellent cathodes for MFCs due to their high catalytic activity, strong stability and low cost.

 Please wait while we load your content...
Please wait while we load your content...