Mechanochemical synthesis of an efficient nanocrystalline BaFBr:Eu2+ X-ray storage phosphor
Abstract
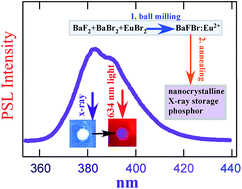
A straightforward and direct preparation route for nanocrystalline BaFBr:Eu2+ by ball-milling is reported. We demonstrate that the as-prepared nanocrystalline powder becomes an efficient photostimulable X-ray storage phosphor upon annealing at temperatures in the range of 400 to 900 °C. The dependence of crystallite size, photoluminescence and storage phosphor properties on annealing temperature and annealing time was investigated and the optimal conditions were determined. After annealing at 900 °C for 1 h, the average crystallite size was found to be ∼200 nm. In comparison with the typical ∼5 μm particles for conventional phosphors, the shape and size distribution of the crystallites may allow higher packing densities, resulting in higher sensitivities, and resolution in computed radiography. The photostimulable storage properties compare favorably with results obtained for a commercial BaFBr(I):Eu2+ imaging plate.

 Please wait while we load your content...
Please wait while we load your content...