Synthesis of a new 18F labeled porphyrin for potential application in positron emission tomography. In vivo imaging and cellular uptake
Ana V. C. Simõesa,
Sara M. A. Pintoa,
Mário J. F. Calvete *a,
Célia M. F. Gomesbc,
Nuno C. Ferreiracd,
Miguel Castelo-Brancocd,
Jordi Llope,
Mariette M. Pereira*a and
Antero J. Abrunhosa*cd
*a,
Célia M. F. Gomesbc,
Nuno C. Ferreiracd,
Miguel Castelo-Brancocd,
Jordi Llope,
Mariette M. Pereira*a and
Antero J. Abrunhosa*cd
aCQC, Department of Chemistry, Rua Larga, 3004-535 Coimbra, Portugal. E-mail: mcalvete@qui.uc.pt; Tel: +351239854474
bPharmacology and Experimental Therapeutics, IBILI - Faculty of Medicine, University of Coimbra, Coimbra, Portugal
cCNC-IBILI Consortium, University of Coimbra, Coimbra, Portugal. E-mail: antero@pet.uc.pt
dInstitute for Nuclear Sciences Applied to Health (ICNAS), University of Coimbra, Coimbra, Portugal
eCICbiomaGUNE, San Sebastián, Spain
First published on 13th November 2015
Abstract
Herein we report, for the first time, the development, labeling optimization and preliminary biodistribution studies of an [18F] radiolabeled meso-tetraphenylporphyrin. After synthesis and characterization of the “cold” fluorinated porphyrin, the conditions have been transferred to an automated radiochemistry module and the desired 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin was prepared in a radiochemical purity >95%. Moreover, data regarding the uptake into human bladder tumor cells and the radiotracer biodistribution after C57BL/6 mice injection are also presented. The maximum cellular uptake was reached at 45 min and was of 2.5%.
1. Introduction
It is widely accepted today that the best way to diminish the worldwide mortality caused by cancer (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per day) is the development of methods for early stage cancer detection, particularly using efficient and economical non-invasive imaging techniques.1 Some of the most important imaging techniques used for cancer diagnosis are: magnetic resonance imaging (MRI)2,3 single photon emission tomography (SPECT)4,5 and positron emission tomography (PET).6,7 PET is a nuclear imaging technique that combines high sensitivity and accurate image quantification allowing the visualization in vivo of chemical processes in organs and tissues that can help the diagnosis of important pathologies. In recent years this technique has also found an increasing application in the development of new drugs by providing critical information regarding in vivo distribution, mechanism of action and kinetics of candidate compounds.8,9
000 per day) is the development of methods for early stage cancer detection, particularly using efficient and economical non-invasive imaging techniques.1 Some of the most important imaging techniques used for cancer diagnosis are: magnetic resonance imaging (MRI)2,3 single photon emission tomography (SPECT)4,5 and positron emission tomography (PET).6,7 PET is a nuclear imaging technique that combines high sensitivity and accurate image quantification allowing the visualization in vivo of chemical processes in organs and tissues that can help the diagnosis of important pathologies. In recent years this technique has also found an increasing application in the development of new drugs by providing critical information regarding in vivo distribution, mechanism of action and kinetics of candidate compounds.8,9
The most commonly used radionuclides for PET are [14O], [13N], [11C], [64Cu], [68Ga] and [18F].10 Among them the most widely used, by far, is [18F], since, contrarily to the complexation of labeled metals, the labeling with [18F] atoms does not significantly alter the biological properties of the molecules and its moderate t1/2 (109.8 min) permits an adequate time for synthetic and imaging processes, allowing its application as a radiotracer outside of the production centre.11 Furthermore, albeit the myriad of applications possible for tetrapyrrolic macrocycles,12 and particularly their ability for accumulation in tumor cells for PDT treatment,13–15 up to now, there are only a few examples in the scientific literature of contrast agents incorporating tetrapyrrolic macrocycles for PET imaging.16 Recently, our group6 presented the optimization of reaction conditions to promote monomethylation of 5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrin with CH3I at minute time scale and the syntheses of the corresponding labeled porphyrin using short-lived carbon-11, prepared in the automated radiolabeling system. The [11C] labeled porphyrin was obtained with high radiochemical purity and specific radioactivity and biodistribution studies were made using PET imaging. Other authors17 reported the synthesis of [64Cu]-5,10,15,20-tetrakis(pentafluorophenyl)porphyrin, through the reaction of freshly prepared [64Cu]CuCl2 with the former, obtaining 97% radiochemical purity with a specific activity of 14–16 GBq mmol−1. However, in the scientific literature, there are very few reports on the labeling of tetrapyrrolic macrocycles with [18F]. For instance, the synthesis of 5-(4-[18F]fluoro-phenyl)-10,15,20-tris(3-methoxyphenyl)porphyrin has been reported,18 where radiolabeled 4[18F] fluorobenzaldehyde was used as starting material and, more recently, other researchers19 published a different approach for the direct [18F]-labeling using [18F] fluoride ion trough nucleophilic substitution reactions with properly substituted zinc porphyrins, with radiochemical yields lower than 10%. Nevertheless, it should be emphasized that none of the reported studies on [18F] labeling presented any biodistribution studies of the radiolabeled compounds.
Herein we describe for the first time the development, labeling and biodistribution studies of a [18F] radiolabeled meso-tetraphenylporphyrin. After synthesis and characterization of the “cold” fluorinated porphyrin, we have proceeded to the synthesis of the desired 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin using an automated radiochemistry module. The complete process including purification and quality control using HPLC is described. Moreover, data regarding the uptake into human bladder tumor cells and the radiotracer biodistribution after C57BL/6 mice injection is also is also presented.
2. Results and discussion
2.1. “Cold” synthesis of fluorinated porphyrin 4
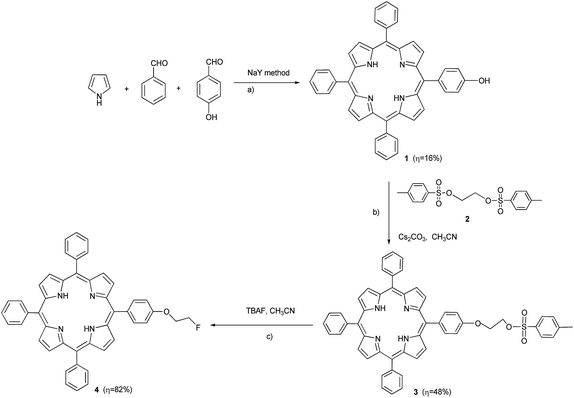
The synthesis of 5-(4-hydroxyphenyl)-10,15,20-triphenylporphyrin (1)20 was carried out using the NaY method, previously described by our group,21 which involves the use of a Al-zeolite as Lewis catalyst in the synthesis of hindered halogenated meso-phenyl porphyrins. Therefore, in a typical reaction, 3 molar equivalents of benzaldehyde, 1 molar equivalent of 4-hydroxybenzaldehyde, 4 molar equivalents of pyrrole mixed in glacial acetic acid and nitrobenzene (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), in a 0.42 molar concentration, and 0.016 molar equivalents of NaY were used.22 The reaction mixture was heated at 130 °C for 2 hours and 1 was obtained, after workup, in 16% yield (Scheme 1a). Then, porphyrin 1 was reacted with 1,2-diyl bis(4-methylbenzenosulfonate)ethane, which was previously prepared according to literature procedures,23 using Cs2CO3 as base, yielding 48% of monotosylated porphyrin 3 (Scheme 1b).
5), in a 0.42 molar concentration, and 0.016 molar equivalents of NaY were used.22 The reaction mixture was heated at 130 °C for 2 hours and 1 was obtained, after workup, in 16% yield (Scheme 1a). Then, porphyrin 1 was reacted with 1,2-diyl bis(4-methylbenzenosulfonate)ethane, which was previously prepared according to literature procedures,23 using Cs2CO3 as base, yielding 48% of monotosylated porphyrin 3 (Scheme 1b).
In order to check the “cold” fluorination of porphyrin 3, to obtain fluorinated porphyrin 4 (Scheme 1c), under laboratorial conditions, the nucleophilic substitution reaction was optimized with two different fluorinating agents, namely tetra-n-butylammonium fluoride (TBAF) and cesium fluoride (CsF), using acetonitrile (CH3CN) as solvent in all experiments (Table 1).
| Entry | Fluorinating agents | T (°C) | Reaction time (h) | Yield of 4 (%) |
|---|---|---|---|---|
| 1 | CsF | 70 | 11 h | 59 |
| 2 | 80 | 8 h | 62 | |
| 3 | 90 | 6 h | 63 | |
| 4 | TBAF | 70 | 2 h | 80 |
| 5 | 80 | 1 h | 80 | |
| 6 | 90 | 1 h | 87 | |
| 7 | 90 | 0.5 h | 85 |
From Table 1, we can assume that the best conditions to promote the nucleophilic substitution reaction using compound 3 involves the use of TBAF as fluorinating agent, since reactions using this agent (entries 4–7, Table 1) reached yields of 80–90%, significantly higher than the ones obtained using CsF as fluorinating agent (entries 1–3, Table 1).
Moreover, while a one hour reaction time at 90 °C produced a yield of 87% (entry 6, Table 1), the substitution reaction carried out, at the same temperature, in 30 minutes yielded compound 4 in 85% yield (entry 7, Table 1). Since the reaction time is a crucial aspect for optimizing the conditions for preparing a suitable [18F] radiotracer (t1/2 [18F] = 109.8 min), we decided that these were the optimal conditions for preparation of compound 4. So, in a typical experiment, the porphyrin 3 was dissolved in CH3CN, after which a THF solution of TBAF was added. The reaction mixture was left to react at 90 °C for 30 min. After workup the desired fluorinated porphyrin 4 (Scheme 1c) was obtained in 85% yield.
In order to acquire the purity and efficiency of the nucleophilic substitution reaction, HPLC apparatus equipped with an analytical column was used to optimize the separation conditions of porphyrins 3 and 4, using the HPLC conditions depicted in Fig. 1.
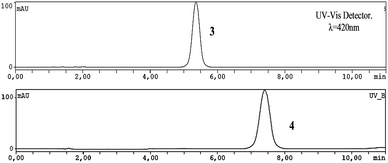 | ||
| Fig. 1 HPLC chromatograms of porphyrins 3 and 4. Conditions: 98% methanol: 2% ammonium formate buffer 0.1 M (pH = 7.2) and 1 mL min−1 flux. | ||
Optimization of separation conditions gave a retention time of 5–6 min for 3, while for fluorinated porphyrin 4 a retention time of 7–8 min was achieved. Moreover, the conditions for HPLC separation using a semi-preparative column were also optimized. In this case, in optimum conditions, the retention time of 3 was 9–10 min (550–650 s) and for 4 a retention time of 13–14 min (800–900 s) was achieved (Fig. 2).
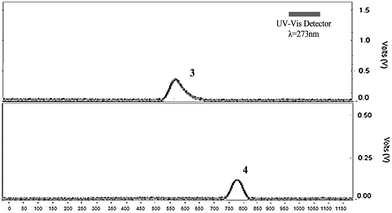 | ||
| Fig. 2 HPLC chromatogram of porphyrins 3 and 4, obtained using a semi-preparative column. Conditions: 98% methanol: 2% ammonium formate buffer 0.1 M (pH = 7.2) and flux of 8 mL min−1. | ||
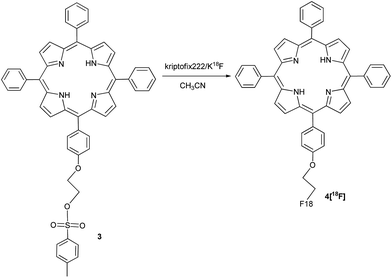
2.2. [18F] radiolabeling: synthesis of porphyrin [18F]-4
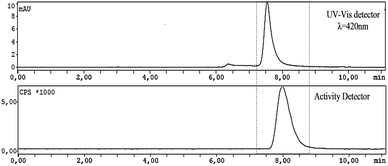
Once checked the “cold” conditions for the synthesis of the fluorinated meso-tetraphenyl substituted porphyrin, the reaction was translated to an automated radiosynthesis process. A modification of the standard Hamacher method was used.24 Aqueous [18F]fluoride was produced by the irradiation of H218O via the 18O(p,n)18F nuclear reaction in a cyclotron. Resolubilization of the aqueous [18F]fluoride was accomplished using Kryptofix222 and K2CO3 in a conical vial and azeotropically removing water with acetonitrile in a stream of nitrogen. Finally, the dried [18F]KF was placed into anhydrous CH3CN, followed by the addition of a solution of porphyrin 3 in CH3CN. Then, the solution was sealed and kept at 100 °C for 25 min (Scheme 2).Quality control of the mixture was performed in a HPLC apparatus, equipped with an analytical column, UV-vis detector and activity detector, using the conditions previously obtained for the corresponding non-labeled fluorinated porphyrin (Fig. 3).
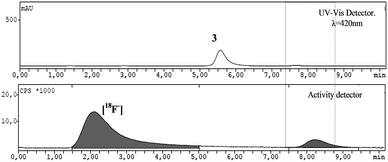 | ||
| Fig. 3 HPLC chromatogram the reaction mixture after radiolabelling with [18F]. Conditions: 98% methanol: 2% ammonium formate buffer 0.1 M (pH = 7.2) and flux of 1 mL min−1. | ||
Through the analysis of the HPLC chromatogram it is possible to attribute the peak at 5–6 min (UV-vis detector) to the starting material (3) and a peak at around 8 min to the labeled fluorinated porphyrin [18F]-4. In addition, the HPLC chromatogram, using the activity detector, shows a peak at 8 minutes attributed to the labeled porphyrin [18F]-4 and another peak at around 2 minutes corresponding to unreacted [18F−] anion. The radiochemical yield was 31.0 ± 15.8% (n = 4). Subsequently, the reaction mixture was purified using an HPLC equipped with the same semi-preparative column under the optimized conditions previously described for the “cold” 5-(2-fluoroethoxyphenyl)-10,15,20-triphenylporphyrin. The fraction corresponding to the labeled porphyrin (showing activity between 10–11 min) (Fig. 4) was collected and re-submitted to quality control to confirm the purity of 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin (Fig. 7).
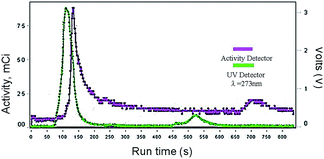 | ||
| Fig. 4 Chromatogram obtained during purification of ([18F]-4) reaction mixture, using a semi-preparative HPLC. | ||
From the analysis of Fig. 5 we observe, both by UV-vis and activity detection, a single peak at ca. 8 min, as previously observed in the cold conditions (Fig. 3) which corroborates the efficient radiosynthesis of porphyrin [18F]-4. Therefore, it should be emphasized that both labeling and purification of porphyrin [18F]-4 with radioactive 18F were successfully accomplished, since the radiolabeled porphyrin was obtained in a radiochemical purity >95%. Specific activity was in the range of 2.7–3.2 Ci μmol−1.
2.3. Tumor cell radiotracer uptake
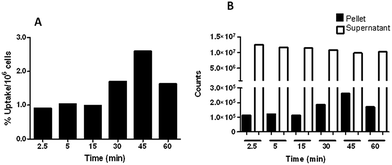
Cellular uptake of 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin ([18F]-4) was carried out in a human bladder cancer cell line (UM-UC3).Cells were incubated with the compound at 37 °C during 60 min. At different time-points, samples were collected and the radioactivity of the pellet and supernatant was measured with a radioisotope calibrator well counter within the 18F sensitivity energy window of 400–600 keV. The results were expressed as the percentage of cell radioactivity associated to the total radioactivity added and normalized per million of cells. The cellular uptake of the compound increased progressively along the first 45 minutes and then started to decline (Fig. 6). The maximum cellular uptake was reached at 45 min and was of 2.5%.
2.4. Exploratory in vivo PET imaging and biodistribution
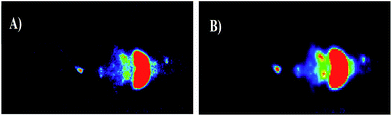
Evaluation of in vivo biodistribution was achieved using normal mice (C57BL/6 mice). The animals were injected at the tail vein with 500 and 900 kBq of 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin ([18F]-4) and placed on a high resolution imaging system. PET planar images of whole body were acquired at 45 minutes (Fig. 7) and a rapid biodistribution, with low uptake in brain, pulmonary and muscle tissues were observed. Uptake in the liver is clearly visible, demonstrating that this is the main pathway for excretion of the compound.3. Conclusions
In summary, we have successfully developed and optimized the conditions for labeling meso-substituted tetraphenylporphyrin derivative with [18F]. After synthesis and characterization of the corresponding “cold” fluorinated porphyrin, the desired 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin was prepared in an automated radiochemistry module with radiochemical purity >95%. Moreover, data regarding the uptake into human bladder tumor cells and the radiotracer biodistribution after C57BL/6 mice injection is also is also presented. The maximum cellular uptake was reached at 45 min and was of 2.5%. Rapid biodistribution, with low uptake in brain, pulmonary and muscle tissues were observed. Uptake in the liver was clearly visible, demonstrating that this is the main pathway for excretion of the compound. Further studies on the improvement on biodistribution and cellular uptake of this type of compounds are needed, and are currently undergoing.4. Experimental
4.1. General considerations
Commercially available reagents were purchased from Aldrich, Fluorochem and Acros, being used as received. All solvents were pre-dried according to standard laboratory techniques. 1H NMR spectra were recorded on a 400 MHz Brucker-Amx. The chemical shifts are given in parts per million (ppm) relative to tetramethylsilane at δ 0.00 ppm for proton spectra. 19F NMR spectra were recorded on a 376.5 MHz Brucker-AmxMass, using TFA (δ = −76.2 ppm) as reference. Mass spectra were acquired using an Applied Biosystems Voyager DE-STR instrument equipped with a nitrogen laser (λ = 337 nm) or Bruker microTOFQ instrument by Unidade de Masas e Proteomica – Universidade de Santiago de Compostela, Spain. Column chromatographies were performed with silica gel grade 60, 70–230 mesh as stationary phase.Radiolabelling with [18F]fluoride was performed at Institute for Nuclear Sciences Applied to Health (ICNAS, University of Coimbra), equipped with an CYCLONE 18/9 cyclotron (IBA. Louvain-la-Neuve, Belgium) and an automated synthesis module (IBA Synthera). Analytical control of the labeling reaction was performed in HLPC Agilent Technologies 1200 series, with an UV-visible detector Agilent Technologies 1200 series with an activity detector Raytest Gabi Star and a chromatographic column Halo C18, 5 μm, 4.6 × 100 mm.
All animal experiments were performed by certified persons (holders of FELASA type C and DGV certificates) according to ethics committee recommendations and approval by the animal research committee of University of Coimbra and Portuguese animal welfare regulation. The researchers always took in consideration the animal suffering and implemented the 3R rule. The experimental procedure used conforms to the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS no. 123) and to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (DHEW Publication No. (NIH) 82–23, Revised 1996, Office of Science and Health Reports, DRR/NIH, Bethesda, MD 20205).
4.2. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH2Cl2 (1
CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent to collect the first fraction (5,10,15,20-tetraphenyl porphyrin) and then with CH2Cl2 to collect the second fraction. This was evaporated to dryness and the resulting solid dried under vacuum and weighed to give 60 mg (0.0938 mmol) of 1 (16% yield). Characterization data is in accordance with the literature.25
3) as eluent to collect the first fraction (5,10,15,20-tetraphenyl porphyrin) and then with CH2Cl2 to collect the second fraction. This was evaporated to dryness and the resulting solid dried under vacuum and weighed to give 60 mg (0.0938 mmol) of 1 (16% yield). Characterization data is in accordance with the literature.251H NMR (400 MHz, CDCl3): δ, ppm 7.73 (d, J = 8.1 Hz, 4H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.34 (d, J = 8.2 Hz, 4H, Ar-
), 7.34 (d, J = 8.2 Hz, 4H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 4.18 (s, 4H, –C
), 4.18 (s, 4H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2), 2.45 (s, 6H, C
2), 2.45 (s, 6H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3). HRMS-ESI-TOF [M + Na]+− m/z 393.0433 calcd [C16H18NaO6S2] 393.0437.
3). HRMS-ESI-TOF [M + Na]+− m/z 393.0433 calcd [C16H18NaO6S2] 393.0437.
UV-vis (toluene): λmax, nm (ε, M−1 cm−1) 420 (3.5 × 105), 515 (2.3 × 104), 546 (6.2 × 103), 591 (6.9 × 103), 656 (4.3 × 103). 1H NMR (400 MHz, CDCl3): δ, ppm 8.85 (s, 8H, β-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 8.22 (d, J = 6.3 Hz, 6H, Ar-
), 8.22 (d, J = 6.3 Hz, 6H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 8.09 (d, J = 8.3 Hz, 2H, Ar-
), 8.09 (d, J = 8.3 Hz, 2H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.95 (d, J = 8.1 Hz 2H, Ar-
), 7.95 (d, J = 8.1 Hz 2H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.79–7.74 (m, 6H, Ar-H + 3H, Ar-H), 7.43 (d, J = 8.0 Hz, 2H, Ar-
), 7.79–7.74 (m, 6H, Ar-H + 3H, Ar-H), 7.43 (d, J = 8.0 Hz, 2H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.15 (d, J = 8.3 Hz, 2H, Ar
), 7.15 (d, J = 8.3 Hz, 2H, Ar![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 4.56 (t, J = 4.0 Hz, 2H, –C
), 4.56 (t, J = 4.0 Hz, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 4.43 (t, J = 4.0 Hz, 2H, –C
2–), 4.43 (t, J = 4.0 Hz, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 2.48 (s, 3H, C
2–), 2.48 (s, 3H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3), −2.76 (s, 2H, N
3), −2.76 (s, 2H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ). HRMS-ESI-TOF [M + H]+. m/z 829.2827 calcd [C53H41N4O4S] 829.2843.
). HRMS-ESI-TOF [M + H]+. m/z 829.2827 calcd [C53H41N4O4S] 829.2843.
UV-vis (toluene): λmax, nm (ε, M−1 cm−1) 420 (3.6 × 105), 514 (2.3 × 104), 545 (6.1 × 103), 591 (6.9 × 103), 656 (4.4 × 103). 1H NMR (400 MHz, CDCl3): δ, ppm 8.84 (s l, 8H, β-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 8.22 (d, J = 6.4 Hz, 6H, Ar-
), 8.22 (d, J = 6.4 Hz, 6H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 8.13 (d, J = 8.4 Hz, 2H, Ar-
), 8.13 (d, J = 8.4 Hz, 2H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.78–7.73 (m, 9H, Ar-
), 7.78–7.73 (m, 9H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 7.30 (d, J = 8.4 Hz, 2H, Ar-
), 7.30 (d, J = 8.4 Hz, 2H, Ar-![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ), 5.01–4.88 (m, 2H, –C
), 5.01–4.88 (m, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), 4.55–4.46 (m, 2H, –C
2–), 4.55–4.46 (m, 2H, –C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–), -2.77 (s, 2H, N
2–), -2.77 (s, 2H, N![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) ). 19F RMN (376.5 MHz, CDCl3): δ, ppm: −222.56 (s, 1F). HRMS-ESI-TOF [M + H]+˙ m/z 677.2721; calcd [C46H34FN4O]: 677.2638.
). 19F RMN (376.5 MHz, CDCl3): δ, ppm: −222.56 (s, 1F). HRMS-ESI-TOF [M + H]+˙ m/z 677.2721; calcd [C46H34FN4O]: 677.2638.
4.3. Radiosynthesis
No-carrier-added aqueous [18F]fluoride was produced in a cyclotron by 18 MeV proton bombardment of 97%-enriched 18O-water via the 18O(p,n)18F nuclear reaction. In an automated synthesis module [18F]fluoride produced (0.8–1.0 GBq) was retained on a ion–exchange column (Sep-Pak Accell Plus QMA from Waters) and then eluted with an acetonitrile solution of Kryptofix® 2.2.2/K2CO3. Water was then removed azeotropically with CH3CN under a stream of N2. To the vial with radioactive mixture, 2 mg of porphyrin (3) was added, previously dissolved in CH3CN, and the reaction left with stirring at 100 °C for 25 minutes.4.4. Cellular uptake studies
The human bladder cancer cell line UMUC3 was purchased from the American Type Culture Collection (ATCC, Rockville, MD) and cultured in RPMI 1640 medium (Gibco, Scotland, UK) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Gibco, Carlsbad, CA), 200 mM of L-glutamine (Sigma) and penicillin–streptomycin (Gibco, penicillin-100 IU mL−1) and streptomycin (100 mg mL−1), at 37 °C in 5% CO2 incubator. For uptake studies, single-cell suspensions (2 × 106 cells per mL) were incubated with 0.75 MBq mL of FDG at 37 °C. At 15, 30 and 60 min samples of 200 μL were taken and transferred to microcentrifuge tubes containing 500 μL of ice-cold PBS and washed twice in PBS. Cell pellets and supernatants were assayed for γ-radioactivity in Radioisotope Calibrator Counter (CRC-15W Capintec, USA) within the 18F sensitivity energy window of 400–600 keV. Results are reported as the percentage of cell-radioactivity associated to the total radioactivity added and normalized per million of cells.4.5. PET imaging
C57BL/6 mice were anesthetized with isoflurane and injected with 500 and 900 kBq of 5-(2-[18F]fluoroethoxyphenyl)-10,15,20-triphenylporphyrin (4[18F]) in the tail vein. Images were acquired after an uptake period of 45 min using a high resolution ClearPEM system (PETsys, Medical PET Imaging Systems, Portugal) with long LYSO crystals and depth-of-interaction information.26 Image reconstruction was performed with interactive reconstruction (5 iterations) with gaussian smoothing at 1.2 mm spatial resolution.Acknowledgements
The authors thank FCT-Portugal and FEDER-COMPETE Programme for funding PEst-OE/QUI/UI0313/2014 and UID/QUI/00313/2013. NMR data was collected at the UC-NMR facility, supported by REEQ/481/QUI/2006, RECI/QEQ-QFI/0168/2012 and CENTRO-07-CT62-FEDER-002012. A. V. C. Simões, S. M. A. Pinto and M. J. F. Calvete thanks SFRH/BD/65699/2009, SFRH/BPD/84619/2012 and SFRH/BPD/99698/2014, respectively, all supported by FCT-Portugal.Notes and references
- X. Chen, Molecular Imaging probes for cancer research, World Scientific Publishing Co. Pte. Ltd., Singapore, 2012 Search PubMed.
- J. Luo, L.-F. Chen, P. Hu and Z.-N. Chen, Inorg. Chem., 2014, 53, 4184 CrossRef CAS PubMed.
- C. F. G. C. Geraldes and S. Laurent, Contrast Media Mol. Imaging, 2009, 4, 1 CrossRef CAS PubMed.
- C. Rangacharyulu and C. K. Roh, J. Radioanal. Nucl. Chem., 2015, 305, 87 CrossRef CAS.
- C.-C. Chang, C.-H. Chang, C.-C. Shen, C.-L. Chen, R.-S. Liu, M.-H. Lin and H.-E. Wang, Bioorg. Med. Chem., 2015, 23, 2261 CrossRef CAS PubMed.
- N. P. F. Gonçalves, A. V. C. Simões, A. R. Abreu, A. J. Abrunhosa, J. M. Dabrowski and M. M. Pereira, J. Porphyrins Phthalocyanines, 2015, 18, 1 Search PubMed.
- S. S. Gambhir, Nat. Rev. Cancer, 2002, 2, 683 CrossRef CAS PubMed.
- M. P. S. Dunphy and J. Lewis, J. Nucl. Med., 2009, 50, 106S CrossRef CAS PubMed.
- E. Fernandes, Z. Barbosa, G. Clemente, A. Rodrigues, F. Alves and A. J. Abrunhosa, Curr. Radiopharm., 2012, 5, 90 CrossRef PubMed.
- A. J. Abrunhosa and M. I. Prata, Radiopharmaceuticals: Development and Main Applications, in Nuclear Medicine Physics, ed. J. J. de Lima, CRC Press, 2010 Search PubMed.
- S. E. Snyder and M. R. Kilbourn, Chemistry of fluorine-18 radiopharmaceuticals, in Handbook of Radiopharmaceuticals: Radiochemistry and Applications, John Wiley & Sons Ltd, 2003 Search PubMed.
- (a) M. Silva, M. J. F. Calvete, M. M. Pereira and H. D. Burrows, RSC Adv., 2013, 3, 22774 RSC; (b) M. Silva, M. J. F. Calvete, N. P. F. Goncalves, H. D. Burrows, M. Sarakha, A. Fernandes, M. F. Ribeiro, M. E. Azenha and M. M. Pereira, J. Hazard. Mater., 2012, 233, 79 CrossRef PubMed; (c) G. Bottari, G. de la Torre, D. M. Guldi and T. Torres, Chem. Rev., 2010, 110, 6768 CrossRef CAS PubMed; (d) M. G. Walter, A. B. Rudine and C. C. Wamser, J. Porphyrins Phthalocyanines, 2010, 14, 759 CrossRef CAS; (e) M. J. F. Calvete, Int. Rev. Phys. Chem., 2012, 31, 319 CrossRef CAS; (f) D. Dini, M. J. F. Calvete, M. Hanack, W. Z. Chen and W. Ji, ARKIVOC, 2006, 3, 77 Search PubMed; (g) D. Dini, M. Calvete, S. Vagin, M. Hanack, A. Eriksson and C. Lopes, J. Porphyrins Phthalocyanines, 2006, 10, 1165 CrossRef CAS; (h) D. Dini, M. Meneghetti, M. J. F. Calvete, T. Arndt, C. Liddiard and M. Hanack, Chem.–Eur. J., 2010, 16, 1212 CrossRef CAS PubMed; (i) C. A. Henriques, N. P. F. Gonçalves, A. R. Abreu, M. J. F. Calvete and M. M. Pereira, J. Porphyrins Phthalocyanines, 2012, 16, 290 CrossRef CAS.
- M. M. Pereira, L. G. M. Arnaut, S. S. Formosinho and C. J. P. Monteiro, New chlorin and/or bacteriochlorin derivatives of porphyrin, useful as anti-cancer and/or antiviral and/or antimicrobic drugs, WO/2006/053707, 2006.
- M. M. Pereira, C. J. P. Monteiro, A. V. C. Simões, S. M. A. Pinto, L. G. Arnaut, G. F. F. Sa, E. F. F. Silva, L. B. Rocha, S. Simões and S. J. Formosinho, J. Porphyrins Phthalocyanines, 2009, 13, 567 CrossRef CAS.
- J. M. Dabrowski, L. G. Arnaut, M. M. Pereira, C. J. P. Monteiro, K. Urbanska, S. Simões and G. Stochel, ChemMedChem, 2010, 5, 1770 CrossRef CAS PubMed.
- M. J. F. Calvete, A. V. C. Simões, C. A. Henriques, S. M. A. Pinto and M. M. Pereira, Curr. Org. Synth., 2014, 11, 127 CrossRef CAS.
- Y. Fazaeli, A. R. Jalilian, M. M. Amini, M. Aboudzadeh, S. Feizi, A. Rahiminezhad and K. Yousefi, J. Radioanal. Nucl. Chem., 2013, 295, 255 CrossRef CAS.
- R. R. Kavali, B. C. Lee, B. S. Moon, S. D. Yang, K. S. Chun, C. W. Choi, C.-H. Lee and D. Y. Chi, J. Labelled Compd. Radiopharm., 2005, 48, 749 CrossRef CAS.
- E. Ranyuk, H. Ali, B. Guérin and J. E. Lier, J. Porphyrins Phthalocyanines, 2013, 17, 850 CrossRef CAS.
- (a) M. J. F. Calvete, J. P. C. Tome and J. A. S. Cavaleiro, J. Heterocycl. Chem., 2014, 51, E202 CrossRef CAS; (b) S. M. A. Pinto, M. A. O. Lourenço, M. J. F. Calvete, A. R. Abreu, M. T. S. Rosado, H. D. Burrows and M. M. Pereira, Inorg. Chem., 2011, 50, 7916 CrossRef CAS PubMed; (c) A. M. V. M. Pereira, A. R. M. Soares, M. J. F. Calvete and G. de la Torre, J. Porphyrins Phthalocyanines, 2009, 13, 419 CrossRef CAS; (d) A. T. Marques, S. M. A. Pinto, C. J. P. Monteiro, J. S. S. de Melo, H. D. Burrows, U. Scherf, M. J. F. Calvete and M. M. Pereira, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1408 CrossRef CAS; (e) S. M. A. Pinto, A. C. B. Neves, M. J. F. Calvete, A. R. Abreu, M. T. S. Rosado, T. Costa, H. D. Burrows and M. M. Pereira, J. Photochem. Photobiol., A, 2012, 242, 59 CrossRef CAS; (f) C. A. Henriques, S. M. A. Pinto, G. L. B. Aquino, M. Pineiro, M. J. F. Calvete and M. M. Pereira, ChemSusChem, 2014, 7, 2821 CrossRef CAS PubMed; (g) C. A. Henriques, S. M. A. Pinto, M. Pineiro, J. Canotilho, M. E. S. Eusébio, M. M. Pereira and M. J. F. Calvete, RSC Adv., 2015, 5, 64902 RSC; (h) M. J. F Calvete, D. Dini, M. Hanack, J. C. Sancho-Garcia, W. Z. Chen and W. Ji, J. Mol. Model., 2006, 12, 543 CrossRef PubMed.
- M. Silva, A. Fernandes, S. S. Bebiano, M. J. F. Calvete, M. F. Ribeiro, H. D. Burrows and M. M. Pereira, Chem. Commun., 2014, 50, 6571 RSC.
- O. Cairon, Phys. Chem. Chem. Phys., 2012, 14, 12083 RSC.
- R. Hoogenboom, M. W. M. Fijten, G. Kickelbick and U. S. Schubert, Beilstein J. Org. Chem., 2010, 6, 773 CrossRef CAS PubMed.
- K. Hamacher, H. H. Coenen and G. Stocklin, J. Nucl. Med., 1986, 27, 235 CAS.
- J. P. C. Tome, M. G. P. M. S. Neves, A. C. Tome, J. A. S. Cavaleiro, A. F. Mendonça, I. N. Pegado, R. Duarte and M. L. Valdeira, Bioorg. Med. Chem., 2005, 13, 3878 CrossRef CAS PubMed.
- M. C. Abreu, J. D. Aguiar, F. G. Almeida, P. Almeida, P. Bento, B. Carrico, M. Ferreira, N. C. Ferreira, F. Goncalves, C. Leong, F. Lopes, P. Lousa, M. V. Martins, N. Matela, P. R. Mendes, R. Moura, J. Nobre, N. Oliveira, C. Ortigao, L. Peralta, R. Pereira, J. Rego, R. Ribeiro, P. Rodrigues, J. Sampaio, A. I. Santos, L. Silva, J. C. Silva, P. Sousa, I. C. Teixeira, J. P. Teixeira, A. Trindade and J. Varela, IEEE Trans. Nucl. Sci., 2006, 53, 71 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |