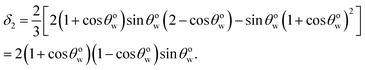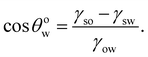DOI:
10.1039/C5RA16096K
(Paper)
RSC Adv., 2015,
5, 80184-80191
Conditions for spontaneous oil–water separation with oil–water separators
Received
10th August 2015
, Accepted 8th September 2015
First published on 8th September 2015
Abstract
Separating oil and water from oil-in-water or water-in-oil systems is immensely important. Such separation is typically achieved through specially fabricated surfaces that demonstrate distinct differences in wettability between water and the specified oil; in other words, these surfaces are either hydrophobic–oleophilic or hydrophilic–oleophobic. While this difference is sufficient to trigger the separation – and this has been the fundamental basis of preparing all surfaces meant for oil–water separation – the spontaneity of the separation process is not a single-valued function of this difference. Considering the free energy change associated with the separation process as an indicator of the extent of the spontaneity of the separation, we show that this spontaneity is additionally dictated by the oil–water interfacial tension. More intriguingly our analysis establishes that for a hydrophobic–oleophilic surface, the separation of oil from oil-in-water systems is more spontaneous than the separation of water from water-in-oil systems, whereas for a hydrophilic–oleophobic surface the separation of oil from oil-in-water systems is less spontaneous than the separation of water from water-in-oil systems.
1 Introduction
The understanding and handling of systems containing a highly stable mixture of oil and water are extremely important for a large number of applications. These mixtures can be in the form of oil-in-water emulsions,1,2 water-in-oil emulsions,3–5 and systems resulting from oil spillage6,7 and the chemical contamination of water. The efficient separation of oil and water in such systems often becomes a necessity. For example, the separation of water by breaking and destabilizing a water-in-oil emulsion is fundamental to ensuring the desired quality of the crude oil that is being produced from production facilities.8 Such breakage of water-in-oil emulsions may also be associated with water harvesting.9 On the other hand, breaking of oil-in-water emulsions becomes a necessity for applications such as enhanced oil recovery.10,11 While these applications are well-known, an invigorated interest in oil–water separation has been aroused by the recent incidents of oil spills worth billions of dollars in damages and the interest in cleaning the oil spills.12–15
One of the most common and the most popular technologies for separating oil and water has been fabricating surfaces that show distinct differences in wettability between water and the chosen oil. In other words, these surfaces are either hydrophobic–oleophilic16–26 or hydrophilic–oleophobic.27–35 Therefore, the fabrication of these surfaces is based on the central hypothesis that the differences in wettability will trigger a difference in the affinity of the individual phases (oil and water) towards these surfaces, which in turn will lead to a spontaneous separation of the oil and water. While this hypothesis is indeed correct (as evidenced by our analysis later), the nature of the separator (i.e., whether it is hydrophobic–oleophilic or hydrophilic–oleophobic) and the nature of the system (oil-in-water or water-in-oil) will strongly dictate the spontaneity of the separation process. This spontaneity, which is a vital factor for dictating how fast the separation occurs, will be central in a large number of applications that depend on the speed of separation (e.g., the separation of water from water-in-crude-oil emulsions). Although several superhydrophobic materials have been fabricated through a combination of rough structure and surface chemistry, and applied to oil–water separation, they are limited to the separation of oil-rich oil–water mixtures but are not suitable for water-rich oil–water mixtures or oil-in-water emulsions.36 A new concept which takes advantage of high-surface-energy materials having water-favoring properties to achieve superoleophobic surfaces, has been used to construct underwater superoleophobic surfaces and shows high separation efficiency with reduced fouling.37 Most remarkably, despite the massive progress in developing more and more efficient technologies and surfaces for oil–water separation, to the best of our knowledge, there has rarely been any mathematical quantification that pinpoints the feasibility (or spontaneity) of oil–water separation in a given system (oil-in-water or water-in-oil) with a given type of separator (hydrophobic–oleophilic or hydrophilic–oleophobic).
The aim of the present study is to provide a free energy based model that quantifies the separation feasibility (in terms of the magnitude of the net free energy change) of different kinds of separators in different kinds of oil–water systems. Our calculations consider two systems: (a) oil drops stabilized in background water and (b) water drops stabilized in background oil. We quantify the manner in which a hydrophobic–oleophilic and a hydrophilic–oleophobic separator works for each of these two cases. Our analysis establishes that the separation in either kind of system with either kind of separator is energetically feasible as long as there is a difference in the wettability of the separator (i.e., the separator is either hydrophobic–oleophilic or hydrophilic–oleophobic) with respect to water and the chosen oil. This establishes the standard hypothesis that has been driving the massive effort in fabricating oil–water separators. More importantly – and this is the central finding of the present study – we establish that the extent of the spontaneity of the separation, in addition to being dictated by the wettability difference of the water and oil on the separator, is also governed by the oil–water interfacial tension, the resulting oil (or water) drop contact angle on the separator in the water (or oil) medium, and the nature of the system (i.e., oil-in water or water-in-oil systems). To be specific, we establish that a hydrophobic–oleophilic separator triggers a much more spontaneous separation of oil from an oil-in-water system as compared to the separation of water from a water-in-oil system. Similarly, a hydrophilic–oleophobic separator is more efficient in separating water from a water-in-oil system as compared to separating oil from an oil-in-water system. To the best of our knowledge, such a quantifiable specification of the separation efficiency of different separators is not known. We believe, therefore, that the present study will motivate a more systematic fabrication of oil–water separation materials based on the systems (oil-in-water or water-in-oil) where these separators are meant to be used.
2 Theory
Here we shall probe the conditions that will ensure a spontaneous oil–water separation by a fabricated separator from either an oil-in-water system or a water-in-oil system. We consider that the contact angles made by a water drop and an oil drop (both in an air medium) on the fabricated surface are θaw and θao, respectively. Please note that the measurement of these angles forms the basis of the fabrication of surfaces for oil–water separation, namely surfaces that simultaneously ensure θaw ≫ π/2 and θao ≪ π/2 (hydrophobic–oleophilic surface) or θaw ≪ π/2 and θao ≫ π/2 (hydrophilic–oleophobic surface). Here we shall study these two cases separately.
Case 1: a hydrophobic–oleophilic surface
For this case, we can write:| |
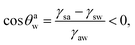 | (1) |
| |
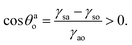 | (2) |
Here γsa, γsw, γaw, γso, and γao are the solid–air, solid–water, air–water, solid–oil, and air–oil surface tensions. Eqn (1) and (2) imply that one must have:
Here we shall test the classical hypothesis that eqn (3) represents sufficient conditions for triggering oil–water separation.
Case 1a: a hydrophobic–oleophilic surface for separating oil from an oil-in-water system. We first consider the case of separating oil from an oil-in-water system by this hydrophobic–oleophilic surface [see Fig. 1(a)]. When this surface is immersed in such an oil-in-water system, oil will spontaneously separate out from the water only when the corresponding change in free energy associated with this separation process is negative. We consider that during this separation, oil drops existing in the form of spheres in the bulk water medium will be deposited on this fabricated surface in the form of spherical caps.
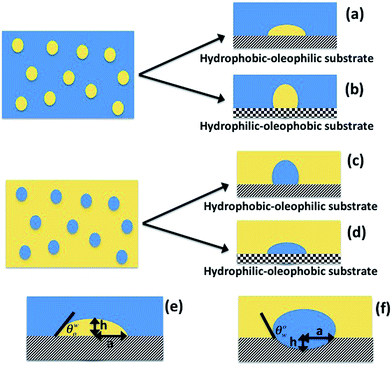 |
| | Fig. 1 Schematic of the oil–water separation. Separating oil from an oil-in-water emulsion by (a) a hydrophobic–oleophilic separator and (b) a hydrophilic–oleophobic separator. Separating water from a water-in-oil emulsion by (c) a hydrophobic–oleophilic separator and (d) a hydrophilic–oleophobic separator. (e) and (f) show the dimensions for cases (a) and (c). | |
Considering the angle formed by the oil drop on this substrate in the water medium is θwo, we may write [using eqn (3)]:
| |
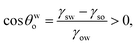 | (4) |
implying
θwo < π/2. Here
γow is the oil–water surface tension. Under these conditions, the energy change associated with the separation of a single oil drop of radius
R can be expressed as:
| | |
ΔU = Uf − Ui = πa2(γso − γsw) + π(a2 + h2)γow − 4πR2γow,
| (5) |
where
a is the base radius and
h is the height of the spherical cap. In
eqn (5), the first term on the right hand side expresses the free energy change on account of the creation of a new solid–oil interface (of area π
a2) and the simultaneous destruction of an existing solid–water interface (of area π
a2), the second term denotes the energy increase on account of the creation of a new oil–water interface [of area equal to the surface area of the spherical cap,
i.e., π(
a2 +
h2)], and the third term expresses the loss of energy on account of the destruction of the oil–water interface (of area 4π
R2) of the spherical oil drops existing in the bulk liquid.
From the consideration of the spherical cap [see Fig. 1(e)], we may write:
| |
h = r(1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo), a = r θwo), a = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo. θwo.
| (6) |
Again since the volumes remain conserved, we can write, using eqn (6),
| |
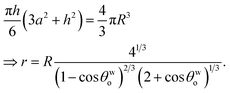 | (7) |
Using eqn (6) and (7) in eqn (5), we can write:
| |
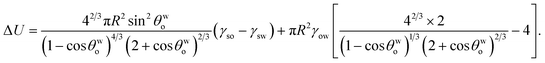 | (8) |
Minimizing eqn (8) with respect to θwo, we can get back eqn (4) (see Appendix A). Therefore eqn (4) is the equilibrium condition. Using eqn (4) in eqn (8), we can finally get the expression for the minimized equilibrium free energy change:
| |
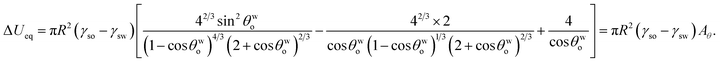 | (9) |
The sign of the parameter Aθ dictates the sign of ΔUeq, and hence the feasibility of the separation of the oil from oil-in-water systems. Since γso < γsw [see eqn (3)], the process is feasible for Aθ > 0 and unfeasible for Aθ < 0.
Case 1b: a hydrophobic–oleophilic surface for separating water from a water-in-oil system. For this case [see Fig. 1(c)], the water drops separate out from the water-in-oil systems; the result is the formation of water spherical caps of contact angle θow on the immersed surface. Therefore, using eqn (3):| |
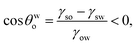 | (10) |
implying π/2 < θow < π. Under these conditions, the energy change associated with the separation of a single water drop of radius R can be expressed as:| | |
ΔU = πa2(γsw − γso) + γow[4πr2 − π(a2 + h2)] − 4πR2γow.
| (11) |
In eqn (11), the three terms on the right hand side denote the same three contributions of the energy change as those for eqn (5). In eqn (11), h is depicted in Fig. 1(f). From the consideration of the spherical cap, we may write:
| |
h = r[1 − cos(π − θow)], a = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(π − θow). sin(π − θow).
| (12) |
Again, since the volumes remain conserved, we can write, using eqn (12):
| |
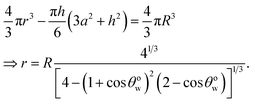 | (13) |
Using eqn (12) and (13) we can rewrite eqn (11) as:
| |
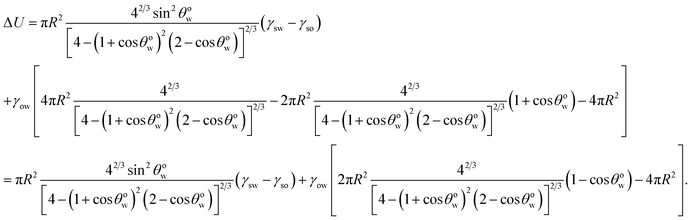 | (14) |
Minimization of eqn (14) yields eqn (10) (see Appendix B). Therefore, eqn (10) is the equilibrium condition. Using eqn (10), we can rewrite eqn (14) to express the minimum equilibrium free energy:
| |
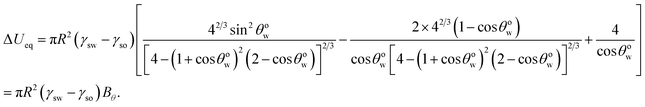 | (15) |
Since (γsw > γso), Bθ < 0 makes the process feasible, whereas Bθ > 0 makes the process unfeasible.
Case 2: a hydrophilic–oleophobic surface
For this case, we can write:| |
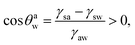 | (16) |
| |
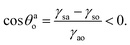 | (17) |
Eqn (16) and (17) imply that one must have:
Case 2a: a hydrophilic–oleophobic surface for separating oil from an oil-in-water system. In this case [see Fig. 1(b)], the oil will separate out from the oil-in-water systems, forming a spherical cap on the hydrophilic–oleophobic substrate. Considering the angle formed by the oil drop on this substrate in the water medium is θwo, we may write:| |
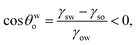 | (19) |
implying π/2 < θwo < π.Using exactly identical calculations as for Case 1b, we can straightaway show:
| | |
ΔUeq = πR2(γso − γsw)B′θ,
| (20) |
where
B′
θ is identical to
Bθ with
θow in
Bθ being replaced by
θwo in
B′
θ.
Case 2b: a hydrophilic–oleophobic surface for separating water from a water-in-oil system. This case is depicted in Fig. 1(d). Considering the angle formed by the water drop on this substrate in the oil medium is θow, we may write:| |
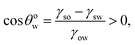 | (21) |
implying θow < π/2.Using exactly identical calculations as for Case 1a, we can straightaway show:
| | |
ΔUeq = πR2(γsw − γso)A′θ,
| (22) |
where
A′
θ is identical to
Aθ with
θwo in
Aθ being replaced by
θow in
A′
θ.
3 Results
Fig. 2 shows the variation of Aθ (or A′θ) with θwo (or θow). For this case, θwo (or θow) represents the contact angle made by the oil (or water) drop on the hydrophobic–oleophilic (or hydrophilic–oleophobic) surface immersed in an oil-in-water (or water-in-oil) system. Consequently, θwo (or θow) will always be less than π/2. We clearly find that Aθ (or A′θ) is positive for all values of θwo (or θow); this establishes that ΔUeq is always negative [see eqn (9) and (22)], indicating a spontaneous separation process. This separation is the separation of oil-drops from an oil-in-water system using a hydrophobic–oleophilic separator or the separation of water drops from a water-in-oil system using a hydrophilic–oleophobic separator. We shall later discuss the degree of spontaneity of this separation process.
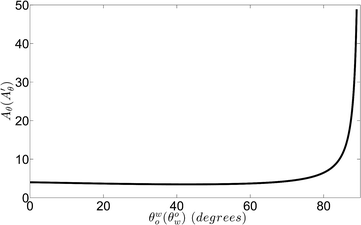 |
| | Fig. 2 Variation of the parameter Aθ (or A′θ) with θwo (or θow). Here θwo (or θow) is the contact angle (<π/2) made by the oil (or water) drop on a hydrophobic–oleophilic (or hydrophilic–oleophobic) surface separating out from an oil-in-water (or water-in-oil) system. | |
Fig. 3 shows the variation of Bθ (or B′θ) with θow (or θwo). For this case, θow (or θwo) represents the contact angle made by the water (or oil) drop on the hydrophobic–oleophilic (or hydrophilic–oleophobic) surface immersed in a water-in-oil (or oil-in-water) system. Consequently, θow (or θwo) will always be more than π/2 but less than π. Bθ (or B′θ) is always negative, implying that ΔUeq is always negative [see eqn (15) and (20)]; as a consequence, the separation process is spontaneous. It is worthwhile to note here that despite the fact that the separating out phase forms a “phobic” contact angle on the separator, ΔU is still negative, confirming that the separation process is spontaneous. This has been the theoretical foundation that has motivated researchers to fabricate oil–water separators as surfaces that simply show a distinct variation in terms of the wettability of oil and water; of course, there is no concrete theoretical foundation as to whether these separators are more appropriate for separating oil from oil-in-water systems or water from water-in-oil systems. We quantify this issue through the free energy change diagram, illustrated in Fig. 4.
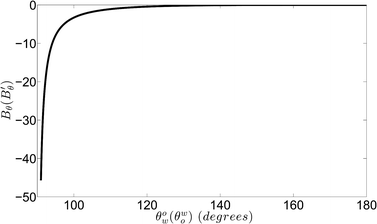 |
| | Fig. 3 Variation of the parameter Bθ (or B′θ) with θow (or θwo). Here θow (or θwo) is the contact angle (>π/2 but <π) made by the water (or oil) drop on a hydrophobic–oleophilic (or hydrophilic–oleophobic) surface separating out from a water-in-oil (or oil-in-water) system. | |
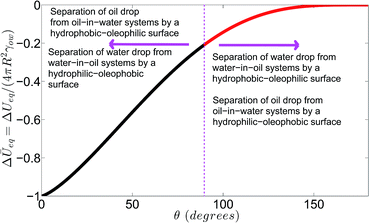 |
| | Fig. 4 Variation of the net change in the dimensionless free energy ΔŪ = ΔU/(4πR2γow) with general θ. Here θ < π/2 for the case where θ = θwo (or θow) for an oil (or water) drop separating out on a hydrophobic–oleophilic (or hydrophilic–oleophobic) separator from an oil-in-water (or water-in-oil) system. Similarly, π/2 < θ < π for the case where θ = θow (or θwo) for a water (or oil) drop separating out on a hydrophobic–oleophilic (or hydrophilic–oleophobic) separator from a water-in-oil (or oil-in-water) system. | |
Fig. 4 shows the variation of the dimensionless minimum equilibrium free energy change associated with the separation process. This is the central result of this paper. The variation of the energy change is studied as a function of the contact angle θ. This θ is the contact angle made by the separating out phase on the immersed separator surface. Therefore this contact angle will be less than π/2 for separating oil (or water) from an oil-in-water (or water-in-oil) system by a hydrophobic–oleophilic (or hydrophilic–oleophobic) separator; on the other hand, this angle will be greater than π/2 but less than π for separating water (or oil) from a water-in-oil (or oil-in-water) system by a hydrophobic–oleophilic (or hydrophilic–oleophobic) separator. The relative magnitude of the free energy change for these different θ values provides a quantification of the extent of the spontaneity of the separation process. To the best of our knowledge, such a quantification of the extent of the spontaneity of oil–water separation by a separator has been missing in the literature. Fig. 4 establishes that the smaller the contact angle θ is, the larger is the magnitude of the energy change, or in other words, the more spontaneous is the separation process. This is extremely vital information, which establishes that a hydrophobic–oleophilic surface is a much superior separator of oil from an oil-in-water system than of water from a water-in-oil system; similarly, a hydrophilic–oleophobic surface is a much more efficient separator of water from a water-in-oil system than of oil from an oil-in-water system. This distinct demarcation of the separation regime and the identification of the corresponding appropriateness of the different separators are vitally relevant for the fabrication of oil–water separators. If a priori knowledge of the potential use of the separator (whether it is used for separating oil from oil-in-water systems or water from water-in-oil systems) is available, our analysis will form the basis of efforts into designing a separator (hydrophobic–oleophilic or hydrophilic–oleophobic). Fig. 4 also establishes that a critical parameter for determining the efficiency of separation is the contact angle formed by the separating out phase on the separator in the medium of the other phase. The crucial issue here is the contact angle formed on the separator not in the air medium, but in the medium of the other phase. The smaller this angle, the more efficient is the separation. Therefore, while fabricating the separators, the test of the contact angle made by a given phase on the separator in the medium of another phase should also be performed. To summarize, therefore, our analysis provides two key elements for the design of oil–water separators: (a) the separator fabricated should ideally be hydrophobic–oleophilic for separating oil from an oil-in-water system or hydrophilic–oleophobic for separating water from a water-in-oil system, and (b) the separator should ideally be tested for determining the contact angle of one phase in another phase.
4 Discussion
Applications of the present theory in practical examples
Here we shall provide a critical assessment of the actual scenarios where our proposed model is directly and indirectly applicable. In addition, we shall highlight the other practical constraints that may serve as more influential factors than the extent of the feasibility of the separation process in dictating the choice of the separator.
Our model is directly applicable to problems where oil–water separation is sought from an emulsified system (e.g., oil-in-water or water-in-oil emulsions) by using a hydrophobic–oleophilic or hydrophilic–oleophobic separator.19,35,36,38,39 This is due to the fact that the free energy changes are calculated based on the assumption that one phase exists as spherical droplets in the background of another phase. In this context, a careful analysis of the existing literature demonstrates that the idea of the appropriateness of the nature of the separator based on the nature of the system (oil-in-water or water-in-oil) is missing; therefore, it is commonplace to find that one uses a hydrophobic–oleophilic separator for separating water from water-in-oil systems19 and a hydrophilic–oleophobic separator to separate oil from oil-in-water systems.35,36,38,39 As we have illustrated, such a selection will not hinder the separation process, and accordingly such a selection of separators has not been deterred. However, the separation process will become less favourable.
While the separation of oil or water from an emulsion (this is effectively the emulsion-breaking process) is central to a large number of applications (e.g., improving the quality of the crude oil or enhanced oil recovery), issues such as the cleaning of oil spills necessitate oil–water separation from systems where the oil exists as immiscible layers on the water. Such a system is not tractable quantitatively by our model; however, qualitative predictions are perfectly feasible. The best separators for such a system are those where the oil will be easily separated out – from our analysis such separators should be hydrophobic–oleophilic. Existing experimental studies16–18,20,21,24–26 clearly indicate that such a consideration (predicted by our analysis) is well accepted; these studies16–18,20,21,24–26 have used hydrophobic–oleophilic separators for separating oils from a system where the oil forms an immiscible layer on the bulk water.
Our model is not applicable to experiments that filter out one phase from another in a mixture of the two phases. Such filtration is often a necessity to obtain clean water from an oily water and/or recover oil from a water-in-oil emulsion system. For such systems it is desirable that the filter should be such that it allows an easy flow of the bulk phase through it, and at the same time retards the wetting of the suspended phase. Therefore, membranes used to filter out oil from an oily water system should ideally be hydrophilic–oleophobic so that the oil is left behind and the water passes through the filter.40 Similarly, membrane filters used to filter out water from a water-in-oil emulsion system should be hydrophobic–oleophilic to retain the water and transport the oil.41–45 Therefore, the principle of the operation of a filter is completely the reverse of the separator (where the preference should be on ensuring a higher wettability of the departing phase), and therefore our model is not appropriate for a filter.
Other critical issues beyond the scope of the present free energy approach
While the energy-based calculations provided here clearly demonstrate the usefulness of a hydrophobic–oleophilic (or hydrophilic–oleophobic) surface for separating oil (or water) from an oil-in-water (or water-in-oil) system, there are certain practical constraints that should be overcome in order to fabricate surfaces led by the predictions of the present theory. For example, separating oil from an oil-in-water emulsion system by using a hydrophobic–oleophilic surface may enhance the formation of “void spaces” (which can be surface nanobubbles46,47) at the water–substrate interface, caused by the repelling action of the solid on the bulk water. The presence of such “void” spaces will certainly reduce the available surface area for the oil drops to “wet” and separate out. On the contrary, it may be practically more feasible (although energetically less feasible, as established by our analysis) to use a hydrophilic–oleophobic (in particular, an underwater oleophobic36,38,39) surface to separate out oil from an oil-in-water system. The proposition of a better theoretical understanding of such a scenario will necessitate the modification of our present model in order to account for the issues that may be triggered on the introduction of a hydrophobic–oleophilic (or hydrophilic–oleophobic) surface inside bulk water (oil). The resulting analysis will require an a priori knowledge of the contents of the bulk phase (e.g., the amount of dissolved air in the bulk water), which in turn decides the behaviour, dynamics, and morphology of the “void” spaces created at the bulk-solvent–substrate interface.
Finally, we would like to point out two key limitations of our model. The first limitation will be important in situations where the drops may deviate from the assumed spherical cap shapes on the separators. Such a situation arises in the case where the drop size is larger than the corresponding capillary length scale (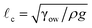 , ρ is the density of the drop and g is the acceleration due to gravity) and gravity flattens the drop making it deviate from the spherical cap shape. The second limitation stems from the disregard of the loss of entropy in our free energy calculations. This loss of entropy is associated with the drops leaving the bulk state (where they are free to move) and depositing as spherical caps on the separator (where they can no longer move). In a future study, we plan to provide a more rigorous model that accounts for these critical limitations.
, ρ is the density of the drop and g is the acceleration due to gravity) and gravity flattens the drop making it deviate from the spherical cap shape. The second limitation stems from the disregard of the loss of entropy in our free energy calculations. This loss of entropy is associated with the drops leaving the bulk state (where they are free to move) and depositing as spherical caps on the separator (where they can no longer move). In a future study, we plan to provide a more rigorous model that accounts for these critical limitations.
5 Conclusions
We have provided here a theoretical formulation pinpointing the extent of the feasibility of oil–water separation by a hydrophobic–oleophilic and hydrophilic–oleophobic separator. Our analysis establishes that a hydrophobic–oleophilic separator is more efficient for separating oil from oil-in-water emulsions, whereas a hydrophilic–oleophobic separator is more useful for separating water from water-in-oil emulsions. As a corollary to this finding, we establish that the usefulness of a separator should not merely be judged by the difference in its wetting behaviour (in an air medium) with respect to water and oil; rather the wettability of one phase in the medium of another phase should also be tested. We believe that our proposed theoretical framework will be helpful for experimentalists designing separators directed towards separation applications in a specified system, i.e., an oil-in-water or water-in-oil system.
A Appendix A: derivation of eqn (4) by minimizing eqn (8) with respect to θwo
Eqn (8) when minimized with respect to eqn (4) yields:| |
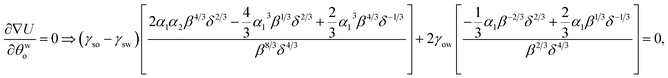 | (A.1) |
where α1 = sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, α2 = cos
θwo, α2 = cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, β = 1 − cos
θwo, β = 1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, and δ = 2 + cos
θwo, and δ = 2 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo. Multiplying all the terms of eqn (A.1) by α1−1β−1/3δ1/3, we can rewrite eqn (A.1) as:
θwo. Multiplying all the terms of eqn (A.1) by α1−1β−1/3δ1/3, we can rewrite eqn (A.1) as:| |
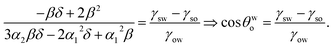 | (A.2) |
B Appendix B: derivation of eqn (10) by minimizing eqn (14) with respect to θow
Eqn (14) when minimized with respect to θow yields (after some algebra):| |
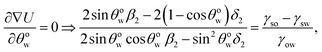 | (B.1) |
where| |
β2 = 4 − (1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow)2(2 − cos θow)2(2 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow) = (1 − cos θow) = (1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow)2(2 + cos θow)2(2 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow), θow),
| (B.2) |
| |
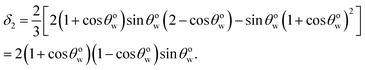 | (B.3) |
Using eqn (B.2) and (B.3) in eqn (B.1), we obtain:
| |
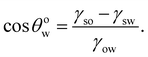 | (B.4) |
References
- E. R. Garrett, J. Pharm. Sci., 1965, 54, 1557 CrossRef PubMed.
- E. Fredrick, P. Walstra and K. Dewettinck, Adv. Colloid Interface Sci., 2010, 153, 30 CrossRef CAS PubMed.
- P. K. Kilpatrick, J. Pharm. Sci., 2012, 46, 4017 Search PubMed.
- M. F. Fingas, J. Pet. Sci. Eng., 2014, 3, 38 CrossRef.
- M. F. Fingas, Spill Sci. Technol. Bull., 1995, 2, 55 CrossRef CAS.
- J. M. Teal and R. W. Howarth, Environ. Manage., 1984, 8, 27 CrossRef.
- B. S. Levy and W. J. Nassetta, Int. J. Occup. Environ. Health, 2011, 17, 161 CrossRef CAS PubMed.
- M. Rondón, P. Bouriat and J. Lachaise, Energy Fuels, 2006, 20, 1600 CrossRef.
- G. Liu, M. Cai, X. Wang, F. Zhou and W. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 11625 CAS.
- M. Li, M. Lin, Z. Wu and A. A. Crhisty, Fuel, 2004, 84, 183 CrossRef PubMed.
- G. J. Hirasaki, C. A. Miller, O. G. Raney, M. K. Poindexter, D. T. Nguyen and J. Hera, Energy Fuels, 2011, 25, 555 CrossRef CAS.
- http://www.npr.org/2015/04/20/400374744/5-years-after-bp-oil-spill-effects-linger-and-recovery-isslow.
- https://www.sciencenews.org/article/five-years-deepwater-horizon-oil-spills-impactlingers.
- S. Kleindienst, J. H. Paul and S. B. Joye, Nat. Rev. Microbiol., 2015, 13, 388 CrossRef CAS PubMed.
- G. M. King, J. E. Kostka, T. C. Hazen and P. A. Sobecky, Annual Review of Marine Science, 2015, 7, 377 CrossRef CAS PubMed.
- E. Wang, H. Wang, Z. Liu, R. Yuan and Y. Zhu, J. Mater. Sci., 2015, 50, 4707 CrossRef CAS.
- Q. Ke, Y. Jin, P. Jiang and J. Yu, Langmuir, 2014, 30, 13137 CrossRef CAS PubMed.
- B. Li, L. Li, L. Wu, J. Zhang and A. Wang, ChemPlusChem, 2014, 79, 850 CrossRef CAS PubMed.
- B. R. Solomon, M. N. Hyder and K. K. Varanasi, Sci. Rep., 2014, 4, 5504 CAS.
- B. Ge, Z. Zhang, X. Zhu, X. Men and X. Zhou, Colloids Surf., A, 2014, 457, 397 CrossRef CAS PubMed.
- F. Liu, M. Ma, D. Zang, Z. Gao and C. Wang, Carbohydr. Polym., 2014, 103, 480 CrossRef CAS PubMed.
- D.-D. Laa, T. A. Nguyen, S. Lee, J. W. Kim and Y. S. Kim, Appl. Surf. Sci., 2011, 257, 5705 CrossRef PubMed.
- Q. Wang, X. Cui, Y. Xiao and Q. Chen, Appl. Surf. Sci., 2007, 253, 9054 CrossRef CAS PubMed.
- L. Wu, J. Zhang, B. Li and A. Wang, J. Colloid Interface Sci., 2014, 413, 112 CrossRef CAS PubMed.
- B. Ge, Z. Zhang, X. Zhu, X. Men, X. Zhou and Q. Xue, Compos. Sci. Technol., 2014, 102, 100 CrossRef CAS PubMed.
- J. Ge, Y. D. Ye, H. B. Yao, X. Zhu, X. Wang, L. Wu, J. L. Wang, H. Ding, N. Yong, L. H. He and S. H. Yu, Angew. Chem., 2013, 53, 3612 CrossRef PubMed.
- P. S. Brown, O. D. L. A. Atkinson and J. P. S. Badyal, ACS Appl. Mater. Interfaces, 2014, 6, 7504 CAS.
- X. Zhu, W. Tu, K.-H. Wee and R. Bai, J. Membr. Sci., 2014, 466, 36 CrossRef CAS PubMed.
- X. Zhu, H.-E. Loo and R. Bai, J. Membr. Sci., 2013, 436, 47 CrossRef CAS PubMed.
- F. E. Ahmed, B. S. Laila, N. Hilal and R. Hashaikeh, Desalination, 2014, 344, 48 CrossRef PubMed.
- T. Darmanin and F. Guittard, J. Colloid Interface Sci., 2013, 408, 101 CrossRef CAS PubMed.
- J. Zeng and Z. Guo, Colloids Surf., A, 2014, 444, 283 CrossRef CAS PubMed.
- L. Zhang, Y. Zhong, D. Cha and P. Wang, Sci. Rep., 2013, 3, 2326 Search PubMed.
- P. S. Brown and B. Bhushan, Sci. Rep., 2014, 5, 8701 CrossRef PubMed.
- A. K. Kota, G. Kwon, W. Choi, J. M. Mabry and A. Tuteja, Nat. Comm., 2012, 3, 1025 CrossRef PubMed.
- W. Zhang, Y. Zhu, X. Liu, D. Wang, J. Li, L. Jiang and J. Jin, Angew. Chem., Int. Ed., 2014, 53, 856 CrossRef CAS PubMed.
- Z. Xue, S. Wang, L. Lin, L. Chen, M. Liu, L. Feng and L. Jiang, Adv. Mater., 2011, 23, 4270 CrossRef CAS PubMed.
- T. Yuan, J. Meng, T. Hao, Z. Wang and Y. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 14896 CAS.
- P.-C. Chen and Z.-K. Xu, Sci. Rep., 2013, 3, 2776 Search PubMed.
- K. Rohrbach, Y. Li, H. Zhu, Z. Liu, J. Dai, J. Andreasen and L. Hu, Chem. Commun., 2014, 50, 13296 RSC.
- C. Lee and S. Baik, Carbon, 2010, 48, 2192 CrossRef CAS PubMed.
- C. H. Lee, N. Johnson, J. Drelich and Y. K. Yap, Carbon, 2011, 49, 669 CrossRef CAS PubMed.
- T. Yu, G. Xu, X. Wang, J. Yang and J. Hu, Bioresources, 2014, 9, 4421 CAS.
- W. Zhang, Z. Shi, F. Zhang, X. Liu, J. Jin and L. Jiang, Adv. Mater., 2013, 25, 2071 CrossRef CAS PubMed.
- A. Asthana, T. Maitra, R. Buchel, M. K. Tiwari and D. Poulikakos, ACS Appl. Mater. Interfaces, 2014, 16, 8859 Search PubMed.
- J. Seddon and D. Lohse, J. Phys.: Condens. Matter, 2011, 23, 133001 CrossRef PubMed.
- X. Zhang and D. Lohse, Biomicrofluidics, 2014, 8, 041301 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 
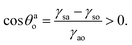
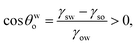
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo), a = r
θwo), a = r![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo.
θwo.
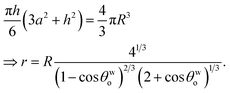
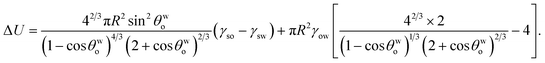
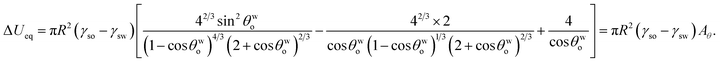
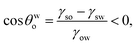
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin(π − θow).
sin(π − θow).
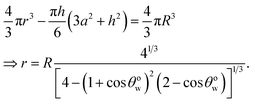
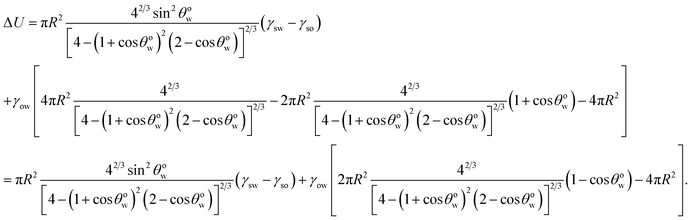

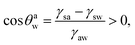
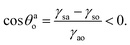
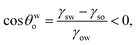
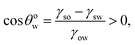
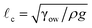 , ρ is the density of the drop and g is the acceleration due to gravity) and gravity flattens the drop making it deviate from the spherical cap shape. The second limitation stems from the disregard of the loss of entropy in our free energy calculations. This loss of entropy is associated with the drops leaving the bulk state (where they are free to move) and depositing as spherical caps on the separator (where they can no longer move). In a future study, we plan to provide a more rigorous model that accounts for these critical limitations.
, ρ is the density of the drop and g is the acceleration due to gravity) and gravity flattens the drop making it deviate from the spherical cap shape. The second limitation stems from the disregard of the loss of entropy in our free energy calculations. This loss of entropy is associated with the drops leaving the bulk state (where they are free to move) and depositing as spherical caps on the separator (where they can no longer move). In a future study, we plan to provide a more rigorous model that accounts for these critical limitations.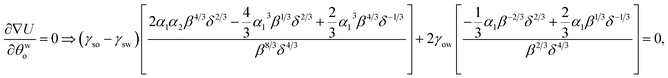
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, α2 = cos
θwo, α2 = cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, β = 1 − cos
θwo, β = 1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo, and δ = 2 + cos
θwo, and δ = 2 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θwo. Multiplying all the terms of eqn (A.1) by α1−1β−1/3δ1/3, we can rewrite eqn (A.1) as:
θwo. Multiplying all the terms of eqn (A.1) by α1−1β−1/3δ1/3, we can rewrite eqn (A.1) as: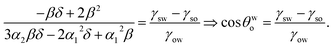
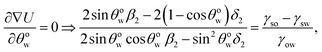
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow)2(2 − cos
θow)2(2 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow) = (1 − cos
θow) = (1 − cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow)2(2 + cos
θow)2(2 + cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θow),
θow),