pH-dependent and self-healing properties of mussel modified poly(vinyl alcohol) hydrogels in a metal-free environment†
Dongjian
Shi
a,
Rongjin
Liu
a,
Weifu
Dong
a,
Xiaojie
Li
a,
Hongji
Zhang
a,
Mingqing
Chen
*a and
Mitsuru
Akashi
b
aThe Key Laboratory of Food Colloids and Biotechnology Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, P. R. China. E-mail: mqchen@jiangnan.edu.cn; Tel: +86-510-85917019
bDepartment of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita 565-0871, Japan
First published on 17th September 2015
Abstract
3,4-Dihydroxyphenylalanine (DOPA)-based polymers are well-known to form functional hydrogels with self-healing properties by chelating metal ions. However, DOPA-based hydrogels with self-healing properties are difficult to obtain in the absence of the metal ions, as previously reported. Thus, the aim of this study is to prepare a self-healable DOPA-based hydrogel in the absence of metal ions. Firstly, poly(vinyl alcohol)–DOPA (PVA–DOPA) was synthesized by modifying PVA with DOPA through an esterification reaction. The composition of the PVA–DOPA polymer was determined by proton nuclear magnetic resonance (1H NMR) spectroscopy. Then, the PVA–DOPA hydrogel in a metal-free environment could be easily prepared by dissolving the polymer in buffer solution. Rheological analyses showed that the PVA–DOPA polymers had different dynamic moduli depending on the pH of the buffer solutions. The results from the FTIR and UV-vis spectra indicated that there were hydrogen bond interactions between the PVA–DOPA polymers under low pH conditions, while there were both hydrogen bond and covalent interactions under high pH conditions. The PVA–DOPA hydrogel could be rapidly self-healed within 270 s, which was much quicker than the hydrogel prepared in the presence of Fe3+ (about 600 s). The metal-free PVA–DOPA hydrogel has the potential for application in coating and biomedical fields.
Introduction
Self-healing gels possess the capability of partially or completely repairing cracks in polymer gels at the macro- or micro-scale and further restoring their original properties and are thus becoming one of the emerging fields in advanced smart materials research.1,2 The self-healing properties of the gels could be developed and regulated by both non-covalent interactions,3–5 including hydrophobic interactions, host–guest interactions, hydrogen bonding, π–π stacking, polymer–nanocomposite interactions and electrostatic interactions, and covalent interactions,6,7 such as those formed by reaction with phenylboronate esters, disulfides and acylhydrazones, and Diels–Alder cycloaddition. Various self-healing gels with soft structures have been developed for proposed application to cell cultures, tissue engineering, drug delivery, coatings, and soft actuators.8–11 For biomedical applications, the self-healing gels are required to have good biocompatibility and nontoxicity. Poly(vinyl alcohol) (PVA) possesses the advantages of low cost, easy manufacture and good biocompatibility.12,13 Yue Zhao’s group14 discovered a self-healing PVA hydrogel using the freezing–thawing method, whose self-healing ability relied on the formation of hydrogen bonds between the hydroxyl groups of the PVA chains by exposure at room temperature without any stimulus or healing agent. Michael W. Keller et al.15 developed a method to sequester an epoxy healing agent within electrospun PVA fibers that could be successfully embedded within a composite material for use in self-healing materials. Jingbin Han’s group16 fabricated hybrid films via the layer-by-layer assembly of layered double hydroxide (LDH) nanoplatelets and poly(sodium styrene-4-sulfonate) (PSS) followed by the subsequent permeation of poly(vinyl alcohol) (PVA), which showed an excellent oxygen barrier performance with humidity-triggered self-healing capability. However, in these examples, the preparation of the PVA self-healing gel was complicated or PVA did not play an important role in the self-healing.3,4-Dihydroxyphenylalanine (DOPA), a catechol derivative, is a synthetic mimic of natural amino acids.17,18 It plays a key role in mussel foot protein adhesion in an aqueous environment. By virtue of the excellent adhesion, DOPA has been demonstrated to have a strong binding affinity to diverse kinds of metal ions or metal oxides (such as Fe3+, TiO2, SiO2) through the formation of hydrogen bonds and metal coordination.19–22 By adjusting the pH from acidic to basic, metal ions could coordinate with DOPA to form mono-, bis-, and tris-catechol/metal complexes with a black color.23,24 Interestingly, the DOPA modified hydrogel showed self-healing properties in the presence of metal ions or metal oxides. However, the DOPA-based hydrogel in the absence of metals did not show self-healing properties.23 Moreover, the obtained DOPA hydrogels were generally black, which limited their application. If a DOPA hydrogel in metal-free conditions has self-healing properties as well as being colorless or a light color, it will be more interesting and useful as an advanced functional material.25
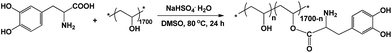
Herein, we describe a strategy to prepare a DOPA-based hydrogel with pH-dependence and self-healing properties in the absence of metal ions. The PVA–DOPA polymer was firstly prepared by modifying PVA with DOPA by an esterification reaction (Scheme 1). Then, the hydrogel was facilely prepared by dissolving the PVA–DOPA polymer in a buffer solution at room temperature. The pH dependent properties and self-healing properties of the hydrogels were investigated. For comparison, a PVA–DOPA/Fe3+ complex hydrogel was also prepared by adding PVA–DOPA into an Fe3+ solution in buffer solution to detect the self-healing properties. The DOPA-based hydrogels without metal ions showed a better performance.
Experimental section
Materials
3,4-Dihydroxyphenylalanine (DOPA, 99%) was obtained from Aladdin Reagent Co., Ltd (Shanghai, China) and used without purification. Poly(vinyl alcohol) (PVA, 1799) was purchased from Sichun Weilun Co., Ltd. Dimethyl sulfoxide (DMSO), sodium bisulfate monohydrate (NaHSO4·H2O), ethanol, acetone, sodium hydroxide (NaOH) and ferric chloride (FeCl3) were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China) and used as received.Synthesis of the DOPA-modified PVA (PVA–DOPA) polymer
PVA (1.76 g, 40 mmol) was dissolved in DMSO (30 mL) at 100 °C. After decreasing the temperature to 80 °C, DOPA (1.97 g, 10 mmol) was added to the PVA solution with NaHSO4·H2O (1.5 g) as catalyst. The reaction was kept at 80 °C for 24 h under N2. The PVA–DOPA polymer was obtained by precipitation in acetone, filtration and washing with acetone three times to remove the DOPA homopolymer. The final white product was dried at 50 °C under vacuum for 24 h. Various PVA–DOPA polymers with different DOPA contents were also synthesized under the same conditions.Preparation of the PVA–DOPA hydrogels
The PVA–DOPA polymers were dissolved in various buffer solutions of pH 3, 7.4, 9, and 12, with the concentration of the PVA–DOPA polymer at about 30%. The PVA–DOPA hydrogels formed within about 1 min after the PVA–DOPA polymer dissolved.Preparation of the PVA–DOPA/Fe3+ hydrogel
The PVA–DOPA polymer (200 mg) was added to a 150 mM FeCl3·6H2O aqueous solution (0.57 mL), and the stoichiometry of DOPA to Fe3+ was 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Then, the PVA–DOPA/Fe3+ complex hydrogel could be prepared by adjusting the pH value to 9 with NaOH.
1. Then, the PVA–DOPA/Fe3+ complex hydrogel could be prepared by adjusting the pH value to 9 with NaOH.
Characterization
UV-vis spectra were recorded on a TU-1901 spectrophotometer (Beijing Purkinje General Instrument Co. Ltd). Fourier transform infrared spectroscopy (FTIR) spectra were recorded by the attenuated total reflection (ATR) method with a FTIR spectrometer (Nicolet iN10, Thermo Fisher Scientific Co., Ltd). Proton nuclear magnetic resonance (1H NMR) spectra were recorded at 400 MHz on a Bruker AVANCE III 400 NMR spectrometer with a sample spinning rate of 5 kHz at 25 °C in D2O. The rheological properties of the various hydrogels were monitored using a rotating rheometer (TA Instruments, DHR-2) at 25 °C. Storage moduli (G′) and loss moduli (G′′) were collected using a parallel plate (25 mm) with a frequency sweep at 25 °C.Results and discussion
Synthesis of the PVA–DOPA polymer
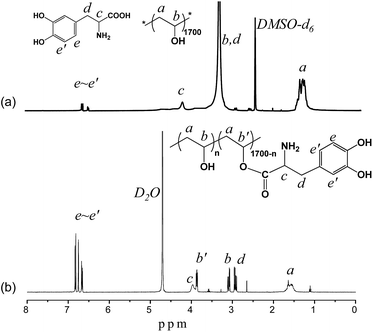
The PVA–DOPA polymer was prepared by an esterification reaction between the hydroxyl groups in PVA and the carboxyl groups in DOPA using NaHSO4·H2O as the catalyst. A series of PVA–DOPA polymers were obtained by changing the molar ratio of DOPA to PVA (Table 1). The 1H NMR spectra of the PVA and DOPA mixture and the PVA–DOPA polymer (PVA–DOPA12 is shown as an example) are shown in Fig. 1. The chemical shifts at 1.5 and 3.2 ppm were assigned to the methylene and methine groups in the PVA chains (Fig. 1a), respectively. The peaks at 4.1, 2.9 and 6.56–6.90 ppm belonged to the methine, methylene and benzene groups in DOPA. After modifying PVA with DOPA, the chemical shift of the methine groups in PVA shifted to 3.8 ppm (Fig. 1b), suggesting the successful synthesis of the PVA–DOPA polymer. By calculation of the peak areas of the methine groups of PVA before and after reaction with DOPA (marked as b and b′ in Fig. 1b), the compositions of PVA–DOPA were obtained, as listed in Table 1. Moreover, the FTIR and UV-vis results also confirmed the chemical structure of PVA–DOPA (Fig. S1 and S2†). Comparing to the specific absorbance of DOPA at 280 nm in the UV-vis spectra, the compositions of PVA–DOPA could also be calculated, and are also listed in Table 1, and were consistent with the results from the 1H NMR spectra.| Sample | PVA(–OH) mmol | DOPA mmol | PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPAa (mol%) DOPAa (mol%) |
PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPAb (mol%) DOPAb (mol%) |
PVA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOPAc (mol%) DOPAc (mol%) |
|---|---|---|---|---|---|
| a Feed molar ratio. b Molar ratio calculated from the 1H NMR spectrum. c Molar ratio calculated from the UV-vis spectrum. | |||||
| PVA–DOPA3 | 12 | 3 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
| PVA–DOPA6 | 12 | 6 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| PVA–DOPA8 | 12 | 8 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 67 67 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 57 57 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51 51 |
| PVA–DOPA10 | 12 | 10 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83 83 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77 77 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
| PVA–DOPA12 | 12 | 12 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82 82 |
100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
Rheological behavior of the PVA–DOPA hydrogels
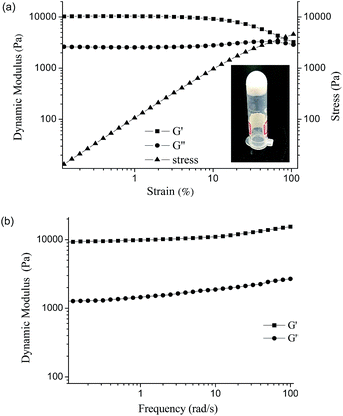
The PVA–DOPA polymer contained many functional groups such as hydroxyl, amine and ester groups. By dissolving the PVA–DOPA polymer in a buffer solution at pH 9 at room temperature (the concentration of PVA–DOPA3 was 30%), the PVA–DOPA hydrogel could be formed with a white color (inset figure in Fig. 2a), due to the hydrogen bond interactions between the hydroxyl, catechol and amine groups. However, the PVA homopolymer without DOPA could not form a hydrogel by this method. Since rheological measurement of a hydrogel is useful for the characterization of its mechanical properties,26 the rheological behavior of the PVA–DOPA hydrogel was evaluated in detail in this study. The linear viscoelastic behavior, constant strain–stress relationship and dynamic moduli including the storage moduli (G′) and loss moduli (G′′) of the PVA–DOPA hydrogel (PVA–DOPA3 as an example) were observed in a strain range with a 6.28 rad s−1 constant frequency at 25 °C, as shown in Fig. 2a. When the strain was ranged from 0.3% to 16%, PVA–DOPA3 showed linear viscoelastic behavior. Then, the frequency sweep experiment of PVA–DOPA3 was performed from low to high frequency (0.1–100 rad s−1) by fixing the strain at 1%, and the results of G′ and G′′ are shown in Fig. 2b. The obtained G′ was around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa and G′′ was 2800 Pa, which is higher than that of the reported DOPA-based gels.23,24G′ is a measure of the elastic energy stored in the network, and thus, a higher G′ value means a stronger elastic network. Accordingly, the PVA–DOPA3 hydrogel formed a relatively high elastic network. Moreover, both G′ and G′′ displayed a relatively weak frequency dependency, indicating that a broad range of relaxation times was involved in the stress relaxation of the associated network.
000 Pa and G′′ was 2800 Pa, which is higher than that of the reported DOPA-based gels.23,24G′ is a measure of the elastic energy stored in the network, and thus, a higher G′ value means a stronger elastic network. Accordingly, the PVA–DOPA3 hydrogel formed a relatively high elastic network. Moreover, both G′ and G′′ displayed a relatively weak frequency dependency, indicating that a broad range of relaxation times was involved in the stress relaxation of the associated network.
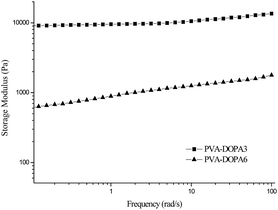
For the PVA–DOPA polymers with various contents of DOPA, the hydrogels were also formed by dissolving the polymers in a buffer solution at pH 9, except for the PVA–DOPA12 polymer. The linear viscoelastic ranges were 0.4–3% for PVA–DOPA6, 0.3–3% for PVA–DOPA8, and 0.5–2.5% for PVA–DOPA10. The G′ values of the formed PVA–DOPA hydrogels were measured by frequency sweep experiments at 1% strain, as shown in Fig. 3. As seen from the results, with the increasing content of DOPA, G′ decreased considerably. The storage modulus is proposed to be directly related to the crosslinking density of the network. Thus, the results indicate that the PVA–DOPA hydrogel with a high DOPA content resulted in a low network crosslinking density. For PVA–DOPA6, the DOPA content was about 30 mol% (Table 1). In pH 9 buffer solution, DOPA molecules easily react with each other.27,28 The higher DOPA content in the PVA chains might induce intramolecular interactions between the DOPA molecules, leading to less intermolecular interactions between chains and a low storage modulus. With a further increase of the DOPA content to 50 mol% and 70 mol% (for PVA–DOPA8 and PVA–DOPA10), G′ decreased almost to 100 Pa. For PVA–DOPA12 with an 80% DOPA content, the intramolecular interactions between the DOPA molecules were too strong and the gel could not form anymore.
For comparison, a hydrogel of the PVA–DOPA polymer and Fe ions was also prepared at pH 9. After adding Fe3+ into the PVA–DOPA polymer, a dark hydrogel was formed (Fig. S3a†), as DOPA could strongly bind to the Fe ions to form a crosslinking-like structure. Resonance Raman spectroscopy illustrated that the chelation interaction between the PVA–DOPA polymer and the Fe ions occurred to form a complex hydrogel (Fig. S3b†). The G′ of the PVA–DOPA3/Fe3+ complex hydrogel was detected to be around 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa at 6.28 rad s−1 (Fig. S3c†), which was higher than that of PVA–DOPA3 (∼10
000 Pa at 6.28 rad s−1 (Fig. S3c†), which was higher than that of PVA–DOPA3 (∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa). There existed hydrogen bonds and coordination interactions (between catechol and Fe) in the complex hydrogel, resulting in more crosslinking bonds. Therefore, the dynamic modulus of the complex hydrogel was higher than that of the PVA–DOPA hydrogel.
000 Pa). There existed hydrogen bonds and coordination interactions (between catechol and Fe) in the complex hydrogel, resulting in more crosslinking bonds. Therefore, the dynamic modulus of the complex hydrogel was higher than that of the PVA–DOPA hydrogel.
pH dependent properties of the PVA–DOPA hydrogels
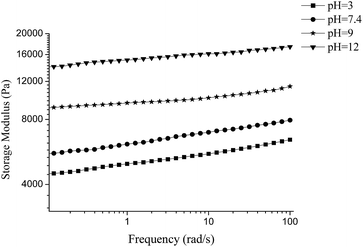
By dissolving the PVA–DOPA polymers in different buffer solutions, the formed hydrogels exhibited different rheological properties. The PVA–DOPA3 hydrogel was used as an example to measure the frequency sweep at 6.28 rad s−1, shown in Fig. 4. In acidic conditions (pH 3), G′ was lowest, around 5500 Pa. G′ increased to 6800 Pa for the hydrogel formed in neutral conditions at pH 7.4. Adjusting the pH to 9 and 12, G′ further increased to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa and 16
000 Pa and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa, respectively. Some of the catechol groups in DOPA were reported to oxidize easily and form o-quinonyl groups in basic solutions, and then DOPA could self-polymerize through the quinonyl and amine groups by Michael addition to form crosslinked structures.27,28 Thus, both hydroxyl and quinonyl groups existed in the DOPA molecules, i.e. both hydrogen bond interactions and Michael additions occurred in the PVA–DOPA hydrogel. On the other hand, the catechol groups could remain stable in acidic solution and could form hydrogen bonds with other functional groups. Accordingly, there were only hydrogen bond interactions in the PVA–DOPA hydrogel under the acidic conditions. A covalent crosslinking bond is stronger than a non-covalent crosslinking bond such as a hydrogen bond, resulting in the dynamic modulus of the PVA–DOPA hydrogel at pH 12 being significantly higher than those at pH 3 and 7.4.
000 Pa, respectively. Some of the catechol groups in DOPA were reported to oxidize easily and form o-quinonyl groups in basic solutions, and then DOPA could self-polymerize through the quinonyl and amine groups by Michael addition to form crosslinked structures.27,28 Thus, both hydroxyl and quinonyl groups existed in the DOPA molecules, i.e. both hydrogen bond interactions and Michael additions occurred in the PVA–DOPA hydrogel. On the other hand, the catechol groups could remain stable in acidic solution and could form hydrogen bonds with other functional groups. Accordingly, there were only hydrogen bond interactions in the PVA–DOPA hydrogel under the acidic conditions. A covalent crosslinking bond is stronger than a non-covalent crosslinking bond such as a hydrogen bond, resulting in the dynamic modulus of the PVA–DOPA hydrogel at pH 12 being significantly higher than those at pH 3 and 7.4.
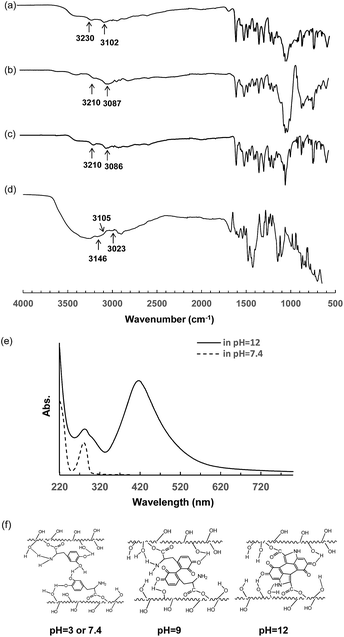
Since the FTIR spectrum of the changes in the stretching of amine groups (νN–H) can determine the intermolecular hydrogen bond interactions occurring between amine and hydroxyl groups,29 FTIR spectra were measured to examine the mechanism of the pH dependent properties of the PVA–DOPA hydrogel. As shown in Fig. 5a, the peaks for the free amine (II) groups for the PVA–DOPA polymer were at 3230 and 3102 cm−1. The peaks clearly red-shifted to around 3210 and 3087 cm−1 for the PVA–DOPA gels formed at pH 3 and 9 (Fig. 5b and c). These prominent red-shifts clearly indicated that there were hydrogen bond interactions between the hydroxyl groups and amine groups via various combinations of H and N or O atoms. For the PVA–DOPA gel formed at pH 12, the amine peak shifted to 3146 and 3023 cm−1 (Fig. 5d). Moreover, there was one shoulder peak at 3105 cm−1, which was assigned to the amine (I) groups by the cycloaddition of DOPA under basic conditions. These results indicated the formation of hydrogen bond and hydrogen and covalent bond interactions in the PVA–DOPA chains depending on the pH values.
To further confirm the structure of DOPA after self-polymerization, UV-vis spectroscopy was also employed to detect the specific adsorption of the PVA–DOPA polymer at pH 7.4 and 12. From the UV-vis spectra (Fig. 5e), a peak at 280 nm that was assigned to the catechol groups appeared in both neutral and basic solutions. By dissolving the PVA–DOPA polymer in a pH 12 solution, a shoulder peak at about 315 nm appeared, suggesting the formation of dehydro-dopamine.30 A new peak at 410 nm belonging to o-quinonyl groups was also observed. These adsorption changes suggested that DOPA was oxidized and self-polymerized to form the quinonyl and crosslinked structure.
According to the above results, we proposed the possible interactions and structures in the formed PVA–DOPA hydrogels, as shown in Fig. 5f. The PVA–DOPA hydrogel was crosslinked via hydrogen bond interactions under the low pH conditions. In weakly basic solution, mostly hydrogen bond interactions and less Michael addition existed in the PVA–DOPA hydrogel, whereas less hydrogen bond interactions and mostly Michael addition existed in the PVA–DOPA gel under strongly basic conditions (Fig. 5f).
PVA–DOPA3/Fe3+ complex hydrogels also have pH responsive properties, as reported in several publications.22,23
Self-healing properties of the PVA–DOPA hydrogels
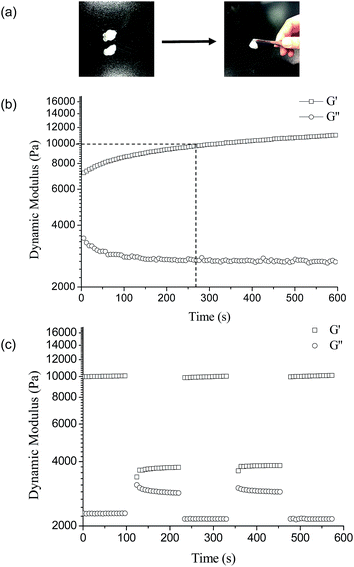
The PVA–DOPA hydrogel was cut into two pieces and then brought back together at the fracture surfaces. The two pieces of the fractured hydrogel healed autonomously and rapidly by contact of the fractured surfaces (Fig. 6a). Moreover, there was no obvious border to be observed between the connected gels after healing. The self-healing properties were also confirmed by rheological measurements. Fig. 6b shows the time-dependent dynamic modulus of the PVA–DOPA3 hydrogel. The time sweep of the PVA–DOPA3 hydrogel was performed after cutting the gel into 9 pieces with a 6.28 rad s−1 constant frequency at 25 °C. The G′ value of the fractured hydrogel firstly decreased from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa to 6500 Pa, whereas G′′ increased to 2900 Pa, as the crosslinked bond was broken. With the healing time, G′ slowly increased and returned to the original value of around 10
000 Pa to 6500 Pa, whereas G′′ increased to 2900 Pa, as the crosslinked bond was broken. With the healing time, G′ slowly increased and returned to the original value of around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa within 270 s. While G′′ correspondingly decreased and reached a balance. These results indicated that the fractured hydrogel was self-healed and returned to the original crosslinking structure, i.e. the PVA–DOPA hydrogel had self-healing properties.
000 Pa within 270 s. While G′′ correspondingly decreased and reached a balance. These results indicated that the fractured hydrogel was self-healed and returned to the original crosslinking structure, i.e. the PVA–DOPA hydrogel had self-healing properties.
The changes of the dynamic moduli G′ and G′′ with the strains were also investigated with a 6.28 rad s−1 constant frequency at 25 °C, as shown in Fig. 6c. When the hydrogel suffered a high strain of 100%, G′ significantly decreased from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa to 3700 Pa and G′′ increased slightly from 2200 Pa to 3000 Pa, suggesting the breaking of the PVA–DOPA structure. Subsequently, changing the strain to 1%, G′ and G′′ rapidly returned to the original values and remained stable. Accordingly, the structure of the hydrogel was recovered and the hydrogel had rapid self-healing properties. Moreover, by re-increasing the strain to 100%, G′ decreased and G′′ increased again. This self-healing could be cycled several times. The mechanism of self-healing is mainly attributed to the dynamically reversible hydrogen bonds between the hydroxyl and amine groups in the PVA and catechol groups. Upon bringing the fractured interfaces back together, the hydroxyl groups and catechol and amine functional groups could interact with each other at the interface to reform the network.
000 Pa to 3700 Pa and G′′ increased slightly from 2200 Pa to 3000 Pa, suggesting the breaking of the PVA–DOPA structure. Subsequently, changing the strain to 1%, G′ and G′′ rapidly returned to the original values and remained stable. Accordingly, the structure of the hydrogel was recovered and the hydrogel had rapid self-healing properties. Moreover, by re-increasing the strain to 100%, G′ decreased and G′′ increased again. This self-healing could be cycled several times. The mechanism of self-healing is mainly attributed to the dynamically reversible hydrogen bonds between the hydroxyl and amine groups in the PVA and catechol groups. Upon bringing the fractured interfaces back together, the hydroxyl groups and catechol and amine functional groups could interact with each other at the interface to reform the network.
The PVA–DOPA hydrogels in different pH solutions also showed self-healing properties. However, the self-healing efficiency of the PVA–DOPA hydrogel at pH 12 was 92% (Fig. S4b†), which was lower than the other PVA–DOPA hydrogels at pH 3 and 9 (around 100%, Fig. 4a and S4a†) after healing for the second time. This lower self-healing was possibly due to the existence of less dynamic hydrogen bond interactions in the PVA–DOPA hydrogel at pH 12, and the covalent bond interactions could not be healed.
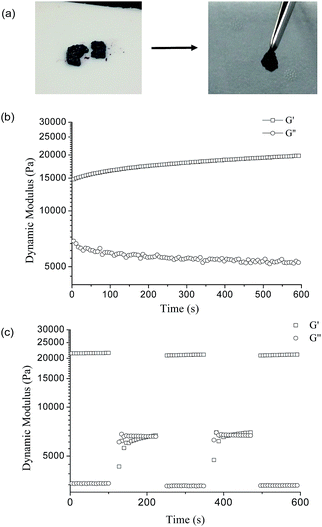
The PVA–DOPA/Fe3+ complex hydrogel also had self-healing properties (Fig. 7a). The self-healing properties of the PVA–DOPA3/Fe3+ complex hydrogel were also confirmed by rheological measurements with a 6.28 rad s−1 constant frequency at 25 °C. After cutting the gel, the G′ value of the PVA–DOPA3/Fe3+ hydrogel decreased to 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa, and G′′ increased to 7000 Pa (Fig. 7b), as the crosslinked bond was broken. During healing, G′ increased and finally returned to 21
000 Pa, and G′′ increased to 7000 Pa (Fig. 7b), as the crosslinked bond was broken. During healing, G′ increased and finally returned to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa, whereas G′′ correspondingly decreased, indicating the hydrogel self-healed to form the original structure. The self-healing time of the PVA–DOPA/Fe3+complex hydrogel was around 600 s, much longer than that of the PVA–DOPA hydrogels. The coordination interactions in the PVA–DOPA/Fe3+ hydrogel might increase the distance between the polymer chains, and then reduce the hydrogen bond interactions between PVA and DOPA. Thus, it needs a longer time to recover. By changing the strain from 1% to 100% during the rheological measurement with a 6.28 rad s−1 constant frequency at 25 °C, the dynamic modulus G′ and G′′ correspondingly vary (Fig. 7c), which was the same as for the PVA–DOPA hydrogel. By combining the fractured gels, the catechol groups and Fe ions should be capable of complexing, as well as healing of the hydrogen bond interactions. The reversible hydrogen bonds and coordination interactions (between catechol and Fe) played a role in the self-healing properties.
000 Pa, whereas G′′ correspondingly decreased, indicating the hydrogel self-healed to form the original structure. The self-healing time of the PVA–DOPA/Fe3+complex hydrogel was around 600 s, much longer than that of the PVA–DOPA hydrogels. The coordination interactions in the PVA–DOPA/Fe3+ hydrogel might increase the distance between the polymer chains, and then reduce the hydrogen bond interactions between PVA and DOPA. Thus, it needs a longer time to recover. By changing the strain from 1% to 100% during the rheological measurement with a 6.28 rad s−1 constant frequency at 25 °C, the dynamic modulus G′ and G′′ correspondingly vary (Fig. 7c), which was the same as for the PVA–DOPA hydrogel. By combining the fractured gels, the catechol groups and Fe ions should be capable of complexing, as well as healing of the hydrogen bond interactions. The reversible hydrogen bonds and coordination interactions (between catechol and Fe) played a role in the self-healing properties.
According to the results, the PVA–DOPA hydrogel showed a more rapid self-healing time and might have wider applications, as compared to the PVA–DOPA/Fe3+ complex hydrogel.
Conclusions
We have designed a DOPA-based hydrogel with self-healing properties in the absence of metal ions. By changing the pH conditions from acidic to basic, the hydrogels showed low to high dynamic moduli, which were induced by the oxidation and self-polymerization of DOPA in the basic solutions. The hydrogels without Fe ions had pH dependent and self-healing properties, and showed more rapid self-healing properties than the hydrogel with Fe ions. The metal-free PVA–DOPA hydrogel has the potential for application in coating and biomedical fields.Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 51173072), the Fundamental Research Funds for the Central Universities (JUSRP51408B), MOE & SAFEA for the 111 Project (B13025), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry.Notes and references
- Z. Wei, J. H. Yang, J. X. Zhou, F. Xu, M. Zrínyi, P. H. Dussault, Y. Osadag and Y. M. Chen, Chem. Soc. Rev., 2014, 43, 8114–8131 RSC.
- H. Chen, X. Ma and H. Tian, Angew. Chem., Int. Ed., 2014, 53, 14149–14152 CrossRef CAS PubMed.
- Y. L. Zhang, L. Tao, S. Li and Y. Wei, Biomacromolecules, 2011, 12, 2894–2901 CrossRef CAS PubMed.
- X. Yang, H. Yu, L. Wang, R. Tong, M. Akram, Y. Chen and X. Zhai, Soft Matter, 2015, 11, 1242–1252 RSC.
- L. Wang, S. Di, W. Wang and S. Zhou, RSC Adv., 2015, 5, 28896–28900 RSC.
- L. He, D. E. Fullenkamp, J. G. Rivera and P. B. Messersmith, Chem. Commun., 2011, 47, 7497–7499 RSC.
- M. Pepels, I. Filot, B. Klumperman and H. Goossens, Polym. Chem., 2013, 4, 4955–4965 RSC.
- H. T. Cui, J. Shao, Y. Wang, P. B. Zhang, X. S. Chen and Y. Wei, Biomacromolecules, 2013, 14, 1904–1912 CrossRef CAS PubMed.
- Y. Huang, P. G. Lawrence and Y. Lapitsky, Langmuir, 2014, 30, 7771–7777 CrossRef CAS PubMed.
- H. Shao, C. F. Wang, J. Zhang and S. Chen, Macromolecules, 2014, 47, 1875–1881 CrossRef CAS.
- P. Zhang, F. Y. Deng, Y. Peng, H. B. Chen, Y. Gao and H. M. Li, RSC Adv., 2014, 4, 47361–47367 RSC.
- H. B. Li, W. K. Zhang, W. Q. Xu and X. Zhang, Macromolecules, 2000, 33, 465–469 CrossRef CAS.
- X. P. Gao, K. Y. Tang, J. Liu, X. J. Zheng and Y. Q. Zhang, J. Wuhan Univ. Technol., 2014, 29, 351–356 CrossRef CAS.
- H. J. Zhang, H. S. Xia and Y. Zhao, ACS Macro Lett., 2012, 1, 1233–1236 CrossRef CAS.
- T. J. Mitchell and M. W. Keller, Polym. Int., 2013, 62, 860–866 CrossRef CAS PubMed.
- Y. B. Dou, A. Zhou, T. Pan, J. Han, M. Wei, D. G. Evans and X. Duan, Chem. Commun., 2014, 50, 7136–7138 RSC.
- J. Sedó, J. Saiz-Poseu, F. Busqué and D. Ruiz-Molina, Adv. Mater., 2013, 25, 653–701 CrossRef PubMed.
- D. J. Shi, R. J. Liu, F. D. Ma, D. Y. Chen, M. Q. Chen and M. Akashi, Chem. Lett., 2014, 43, 959–961 CrossRef CAS.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- S. Moulay, Polym. Rev., 2014, 54, 436–513 CrossRef CAS PubMed.
- M. S. Menyo, C. J. Hawker and J. H. Waite, Soft Matter, 2013, 9, 10314–10323 RSC.
- J. Yu, W. Wei, M. S. Menyo, A. Masic, J. H. Waite and J. N. Israelachvili, Biomacromolecules, 2013, 14, 1072–1077 CrossRef CAS PubMed.
- N. Holten-Andersen, M. J. Harrington, H. Birkedal, B. P. Lee, P. B. Messersmith, K. Y. C. Lee and J. H. Waite, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 2651–2655 CrossRef CAS PubMed.
- M. Krogsgaard, M. A. Behrens, J. S. Pedersen and H. Birkedal, Biomacromolecules, 2013, 14, 297–301 CrossRef CAS PubMed.
- B. K. Ahn, D. W. Lee, J. N. Israelachvili and J. H. Waite, Nat. Mater., 2014, 13, 867–872 CrossRef CAS PubMed.
- F. V. V. Manakker, N. Morabit, C. F. Nostrum and W. E. Hennink, Langmuir, 2008, 24, 12559–12567 CrossRef PubMed.
- A. Postma, Y. Yan, Y. Wang, A. N. Zelikin, E. Tjipto and F. Caruso, Chem. Mater., 2009, 21, 3042–3044 CrossRef CAS.
- A. A. R. Watt, J. P. Bothma and P. Meredith, Soft Matter, 2009, 5, 3754–3760 RSC.
- P. G. Song, Z. G. Xu, Y. Lu and Q. P. Guo, Macromolecules, 2015, 48, 3957–3964 CrossRef CAS.
- P. Y. Sun, J. Wang, X. Yao, Y. Peng, X. Tu, P. Du, Z. Zheng and X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 12495–12504 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR and UV-vis spectra of the PVA–DOPA polymers. Dynamic modulus of the PVA–DOPA/Fe3+ complex hydrogel. See DOI: 10.1039/c5ra15991a |
| This journal is © The Royal Society of Chemistry 2015 |