A high performance ceria-based solid oxide fuel cell operating on underground coal gasification gas
Abstract
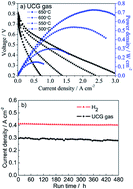
This paper investigates the equilibrium species and theoretical electromotive forces (EMF) of cells fed with 3% H2O humidified simulated underground coal gasification (UCG) gas, and the performance of an anode-supported NiO-Gd0.1Ce0.9O2−δ (GDC)‖GDC‖Ba0.9Co0.7Fe0.2Nb0.1O3−δ (B0.9CFN) cell operated with the UCG gas. The results show that EMF values are always above 1.05 V, and the UCG gas fed cell exhibits maximum power densities of 0.151, 0.299, 0.537 and 0.729 W cm−2 at 500, 550, 600 and 650 °C, respectively, slightly inferior to that of hydrogen fed cell of 0.330, 0.544, 0.765 and 0.936 W cm−2, respectively, as a result of the sluggish reaction kinetics of the UCG gas at the anode. However, the synergy between slow anode reaction kinetics and the sufficient and fast migration of oxygen ions to the anode, attributed to the using of GDC electrolyte and B0.9CFN cathode, suppresses carbon deposition at intermediate temperatures. Only a slight current density decrease ranging from 0.2959 to 0.2790 A cm−2 is observed during the 480 h of durability testing under a constant 0.7 V output voltage at 600 °C for the UCG gas fed cell. The subsequent SEM inspection of the tested cell indicated no measurable carbon deposition on the anode surface, thereby demonstrating that UCG gas is a promising fuel for utilization in SOFCs.

 Please wait while we load your content...
Please wait while we load your content...