Visible-light responsive electrospun nanofibers based on polyacrylonitrile-dispersed graphitic carbon nitride†
Abstract
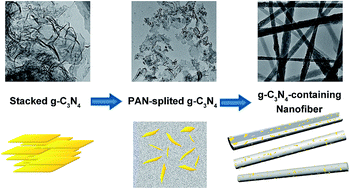
A visible-light responsive photocatalyst, polyacrylonitrile-dispersed graphitic carbon nitride nanofibers (g-C3N4/PAN nanofibers), was synthesized by electrospinning. The g-C3N4 is dispersed uniformly in the nanofibers, which helps it overcome the defects of easy aggregation and difficult recycling of powder catalysts. The model substrate, rhodamine B (RhB), could be adsorbed rapidly into the PAN nanofibers and decomposed efficiently in situ simultaneously in the presence of the g-C3N4 over a wide pH range under visible light irradiation. As a fibrous catalyst, the g-C3N4/PAN nanofibers were quite simple to recycle, and the catalytic activity maintained a high level without obvious decline after being reused several times. In addition, based on the intermediates detected by ultra performance liquid chromatography-mass spectrometry and gas chromatography-mass spectrometry, N-de-ethylation chromophore cleavage and ring-opening mineralization are the main processes in RhB degradation. Finally, a possible mechanism was proposed, in which the hole along with the superoxide radical mainly contribute to the oxidative degradation of RhB.

 Please wait while we load your content...
Please wait while we load your content...