Application of the central composite design to optimize the calcium carbonate-HPMC co-processed excipient prepared by co-spray drying
JinZhi Liab,
Xiao Lin*a,
Fei Wu*ab,
Lan Shena,
YouJie Wangb and
Yi Fengb
aCollege of Chinese Materia Medica, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China. E-mail: duotang@163.com
bEngineering Research Center of Modern Preparation Technology of TCM of Ministry of Education, Shanghai University of TCM, Shanghai 201203, China. E-mail: a1983d3891h@126.com
First published on 27th October 2015
Abstract
To develop a novel co-processed tablet excipient based on calcium carbonate and hydroxypropyl methylcellulose (HPMC), the central composite design-response surface methodology (CCD-RSM) was applied to optimize the amount of HPMC and the solid content of feed suspensions used for co-spray drying by choosing powder and tablet properties as the evaluation indicators. Significant and adequate regression models were developed for each property. An increase in % HPMC improved powder bulk density, tablet tensile strength, and various tableting parameters. However, a high content of HPMC had a negative influence on tablet disintegration time. In addition, the median particle size of the product increased with both factors. However, they had little effect on the fast elastic stretch of tablets. Numerical optimization determined the optimal parameters as HPMC in the feed solid: 6.7% (w/w) and the solid content of the feed: 42% (w/v). The experimental values of the optimized co-processed excipient were mostly close to the model-predicted values. The tablet tensile strength and disintegration time were 3.28 MPa and 8.91 min, respectively. The combined use of CCD, RSM, and the desirability functions can provide an insight into the object studied and the results achieved are helpful for further development of novel co-processed excipients.
1. Introduction
Tablets are still the most commonly used dosage form because of the ease of manufacturing, convenience in administration, accurate dosing, and excellent stability. However, tablet manufacturing has changed significantly over the years because of the introduction of direct compaction and high speed machines, with these two developments increasing the functionality demand on the excipients used in terms of flowability and compatibility.1 Single-component excipients do not always provide requisite performances or physicomechanical properties to allow active pharmaceutical ingredients to be formulated or manufactured successfully. Therefore, co-processed multi-component excipients were brought in.2–5 A co-processed excipient is a combination of two or more established excipients designed to physically modify their properties in a manner not achievable by simple physical mixing. Many co-processing methods can be used, among which spray drying is the most widely used and successful one. A majority of commercially available co-processed excipients are produced by spray drying.3–5Calcium carbonate (CC), employed as a pharmaceutical excipient, is mainly used in solid dosage forms as a diluent6–8 and also used as a calcium supplement, which is inexpensive, easily obtained, and widely used. However, CC is cohesive, and thus, has poor flowability. Moreover, it is a brittle material, which makes it exhibit relatively poor binding properties and low compactibility.9,10 To combat these shortcomings, several CC-based co-processed excipients therefore have been developed with some success, such as Barcroft® CS90 (90% CC-10% starch), ForMaxx® (70% CC-30% sorbitol), and MCC-CC (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20).4 All of these are produced by spray drying. However, to our knowledge, there is no co-processed excipient based on CC and hydroxypropyl methylcellulose (HPMC) yet.
20).4 All of these are produced by spray drying. However, to our knowledge, there is no co-processed excipient based on CC and hydroxypropyl methylcellulose (HPMC) yet.
In direct tableting processes, a dry binder is added generally prior to compaction in order to increase the compactibility of the formulation, and hence, the tensile strength of the resulting tablet.11 Hydroxypropyl methylcellulose (HPMC) is commonly used as a binder in tablets. It is also a common material in thin-film coating.9 With relatively low hygroscopicity and high glass transition temperature, HPMC was found to be able to reduce particle adhesion, and thus, improve production yield during spray drying.12
In our preliminary experiments, the feasibility of improving both powder and tablet properties of CC by co-spray drying with a small amount of HPMC was tested. The results show that most powder properties (e.g., flowability, compactibility, and hygroscopicity) and tableting properties (e.g., yield pressure, tensile strength, and unit effective compaction work) of the co-processed product were significantly improved compared to their physical mixture. In addition, it was reported that % HPMC and the solid content of feeds could affect the performance of spray-dried composite particles.13,14 Thus, in this study, to obtain the ideal CC-HPMC co-spray dried powders, the central composite design-response surface methodology (CCD-RSM) was applied to optimize the amount of HPMC E3 and the solid content of the feed used for co-spray drying. The reason for using HPMC E3, the lowest viscosity grade in the HPMC family, was to minimize the possible adverse effect of HPMC on tablet disintegration time.
CCD-RSM is a preferred method to further optimize the preparation with a nonlinear model to fit the experimental data.15–17 Compared with commonly used traditional methods, such as orthogonal design and uniform design, CCD-RSM has higher accuracy, and can find the optimal value in the whole area. CCD-RSM has been widely applied in food18,19 and pharmaceutical20,21 fields. The objective of the method is to simultaneously optimize the levels of the variables studied to achieve the best systemic performance. In this study, a five-level and two-factor CCD-RSM was used to optimize the amount of HPMC and the solid content of the feed used for co-spray drying by choosing various powder and tablet properties as the evaluation indicators.
2. Materials and methods
2.1. Materials
Calcium carbonate (CC) (Daheng calcium carbonate Development Co., Ltd, Yidu, China), hydroxypropyl methylcellulose (HPMC; Methocel E3, Dow Chemical, Midland, MI, USA), cross-linked polyvinylpolypyrrolidone (PVPP; Kollidon CL, BASF, Ludwigshafen, Germany), magnesium stearate, and acetone (Sinopham Chemical Reagent Co, Shanghai, China) were used as supplied.2.2. Preparation of the spray dried particles
CC was dispersed homogeneously in water solution of HPMC to form the feed dispersion. With constant stirring, the feed dispersion was spray-dried immediately using a pilot scale spray dryer (Mobile Minor 2000, Niro, Søborg, Denmark) with a rotary atomizer operated under the following conditions: inlet temperature, 170 °C; atomizing pressure, 4.0 MPa; and feed rate, ∼22 mL min−1. The dryer was allowed to reach steady state by operating it for at least 30 min with water spraying before the feed was sprayed. The particles collected were sealed into plastic bags and stored in desiccators at room temperature until further tests.2.3. Experimental design
Preliminary experiments were carried out to establish appropriate ranges for the amount of HPMC in the feed solid and the solid content of the feed suspension. The lower limit of the solid content of the feed (19%, w/v) was chosen to obtain a minimum production capacity. And, the upper limit (44%, w/v) was selected to avoid pumping problems and blocking of the atomization device due to high viscosity of suspensions. On the other hand, the maximum amount of HPMC in the feed solid was set as 10.5% (w/w), based on the improvement efficiency and the consideration that further increasing the amount of HPMC could cause unacceptable adverse influence on tablet disintegration time.Table 1 displays the coded and uncoded levels of the two independent variables along with their ranges, which were determined from preliminary trials, and then the central composite design (CCD) was applied to investigate the effects of independent variables on the powder properties (flowability, median particle size, bulk density, moisture content, production yield) and tablet properties (tensile strength, disintegration time, and tableting parameters) of spray-dried particles. The names and symbols of the properties studied are shown in Table 2.
| Independent variables | Ranges | Levels of variables | ||||
|---|---|---|---|---|---|---|
| −1.414 | −1 | 0 | 1 | 1.414 | ||
| A: HPMC in the feed solid (%, w/w) | 3.5–10.5 | 3.5 | 4.5 | 7.0 | 9.5 | 10.5 |
| B: Solid content of the feed (%, w/v) | 19–44 | 19 | 23 | 32 | 40 | 44 |
| Name | Symbol |
|---|---|
| Median particle size | D(0.5) |
| Production yield | PY |
| Moisture content | MC |
| Bulk density | ρb |
| Angle of repose | AR |
| Tensile strength | TS |
| Disintegration time of tablets containing the coprocessed excipient only | DT |
| Disintegration time of tablets composed of 96.5% the coprocessed excipient and 3.5% PVPP | DTP |
| Yield pressure | Py |
| Fast elastic stretch | FES |
| Unit effective compaction work | Esp |
Each factor was coded at five levels according to the following equation.22
 | (1) |
In this study, the design matrix was generated by the Design-Expert® (Version 8.0.6.1) software. The total runs of the CCD were 13, which included one run for each of 4 points from a two-factor two-level full factorial 2λ = 2 design (−1 and +1 being the coded levels for each factor) and 4 additional axial points (2 experimental points per factor at a distance α = (2λ)0.25 = 1.414 from the design center) and five replicated runs for the central point (Table 3). Replication allowed to determine experimental error and to increase the precision of estimates.
| Run | Code | Value | ||
|---|---|---|---|---|
| A (%) | B (%) | A (%) | B (%) | |
| a A, HPMC in the feed solid (%, w/w); B, the solid content of the feed (%, w/v). | ||||
| 1 | 1.414 | 0 | 10.5 | 32 |
| 2 | 0 | 0 | 7.0 | 32 |
| 3 | −1 | −1 | 4.5 | 23 |
| 4 | 1 | −1 | 9.5 | 23 |
| 5 | 0 | −1.414 | 7.0 | 19 |
| 6 | 0 | 0 | 7.0 | 32 |
| 7 | 0 | 0 | 7.0 | 32 |
| 8 | 0 | 0 | 7.0 | 32 |
| 9 | 1 | 1 | 9.5 | 40 |
| 10 | 0 | 0 | 7.0 | 32 |
| 11 | −1 | 1 | 4.5 | 40 |
| 12 | −1.414 | 0 | 3.5 | 32 |
| 13 | 0 | 1.414 | 7.0 | 44 |
To avoid bias, all the experiments were performed in random order. The empirical second order polynomial model was shown as follows:
 | (2) |
The experimental data were analyzed by multiple regression analysis through the generalized least square. Analysis of variance (ANOVA) was used to estimate the statistical parameters. The Design-Expert software was utilized for the response surface analysis. The significant model was used for fitting the response. The lack-of-fit test and a normal probability plot of the residuals were performed in order to evaluate the model and to detect outliers. The models provide several comparative measures for model selection.
2.4. Characterization of particles and tablets
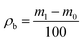 | (3) |
 | (4) |
The works during compaction were also recorded by the press. The Esp value was calculated using eqn (5):
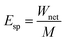 | (5) |
 | (6) |
 | (7) |
3. Results and discussion
Co-spray drying uses a one-step continuous process to dry and agglomerate multi-component powders, which are generally homogeneous, subglobular, and porous, with improved physicochemical and mechanical properties. In addition, it is a technique that can be easily automated, scaled up, and equipped for in-line product analysis. These features altogether offer obvious benefits for the development of composite materials with various applications.28–32In our preliminary experiments, co-spray drying of calcium carbonate (CC) suspension with dissolved hydroxypropyl methylcellulose (HPMC) had demonstrated the efficiency of HPMC to improve various powder and tableting properties of CC.28 The results showed that co-spray drying with HPMC led to a remarkable improvement in flowability for CC, and via reducing particle adhesion, achieved a production yield as high as 81.2%. The tensile strength of tablets made from the co-spray dried product was several times higher than that from their physical mixture. The co-spray dried product also exhibited lower lubrication sensitivity and appropriate tablet disintegration time. It was reported that the content of binders and the solid content of feeds probably affected the performance of the spray dried composite particles by virtue of influencing their size and bulk density, and the distribution of the components.13,14 In light of the above, the central composite design-response surface methodology (CCD-RSM) was therefore carried out in this study to investigate the effects of two factors, i.e., % HPMC and the solid content of the feed, on the powder and tableting properties of the co-spray dried product. The chosen responses were powder flowability, median particle size, bulk density, moisture content, production yield, tablet tensile strength and disintegration time, and tableting parameters (including fast elastic stretch, yield pressure, and Esp).
3.1. Summary statistics for the models
Based on the design matrix of CCD for two factors at five levels, a total of 13 experiments were performed and the observed responses are summarized in Tables 4 and 5. RSM was used to identify the optimum levels of the variables that had significant effects on powder and tablet properties of the co-spray dried product. All the responses observed for the 13 products prepared were fitted to various models using the Design-Expert software. It was observed that the best-fit model was the quadratic or cubic model for each response. Analysis of variance of the responses (Table 6) indicated that for the optimization of the co-spray dried excipient, the surface models developed for powder flowability, median particle size, bulk density, tablet tensile strength, disintegration time, yield pressure, Esp, and fast elastic stretch were significant, without significant lack of fit. In addition, the model summary statistics for the selected significant models were also detailed in Table 6. It can be observed that for powder flowability, median particle size, bulk density, tablet tensile strength, disintegration time, yield pressure, and Esp, R2, predicted R2, and adjusted R2 were in good agreement, resulting in reliable models. Although the response surface model for fast elastic stretch was significant, R2, predicted R2, and adjusted R2 were not in good agreement. Thus, the developed regression model for fast elastic stretch did not show acceptable statistical measures. Moreover, fast elastic stretch values of all design runs were within acceptable ranges. However, for the moisture content, since the ability to spray dry a product to a specific moisture content at a given outlet drying air temperature depends upon the humidity of the air leaving the drying chamber, daily changes of ambient humidity conditions could affect the moisture content in the spray dried powder.33 As a result, the model estimating the moisture content of spray dried powders was not significant. For the production yield, it is mainly affected by spray drying parameters (atomizing air pressure, inlet and outlet drying air temperatures, and feed rate). Thus, both of the factors have little effect on it. In addition, the anti-adhesion effect of HPMC has already approached the maximum when its amount was 3.5%. As a result, the model estimating the production yield was also not significant.| Run | A (%) | B (%) | D(0.5) (μm) | PY (%) | MC (%) | ρb (g mL−1) | AR (°) |
|---|---|---|---|---|---|---|---|
| a Standard deviation.b A, HPMC in the feed solid (%, w/w); B, the solid content of the feed (%, w/v). D(0.5), median particle size; PY, production yield; MC, moisture content; ρb, bulk density; AR, angle of repose. | |||||||
| 1 | 10.5 | 32 | 39.61 (0.01)a | 81.21 | 0.67 (0.02) | 0.298 (0.04) | 33 (0.31) |
| 2 | 7.0 | 32 | 29.50 (0.02) | 79.05 | 0.53 (0.04) | 0.379 (0.01) | 32 (0.38) |
| 3 | 4.5 | 23 | 21.98 (0.04) | 83.25 | 0.37 (0.04) | 0.498 (0.08) | 31 (0.28) |
| 4 | 9.5 | 23 | 34.41 (0.02) | 83.52 | 0.53 (0.05) | 0.308 (0.09) | 31 (0.33) |
| 5 | 7.0 | 19 | 25.97 (0.05) | 80.09 | 0.66 (0.02) | 0.362 (0.06) | 32 (0.23) |
| 6 | 7.0 | 32 | 29.89 (0.01) | 79.98 | 0.56 (0.06) | 0.365 (0.11) | 32 (0.27) |
| 7 | 7.0 | 32 | 29.42 (0.03) | 80.13 | 0.56 (0.03) | 0.372 (0.05) | 31 (0.21) |
| 8 | 7.0 | 32 | 30.04 (0.01) | 79.51 | 0.55 (0.02) | 0.374 (0.01) | 32 (0.31) |
| 9 | 9.5 | 40 | 40.85 (0.06) | 73.50 | 0.51 (0.03) | 0.341 (0.04) | 33 (0.34) |
| 10 | 7.0 | 32 | 29.07 (0.01) | 80.08 | 0.47 (0.06) | 0.352 (0.07) | 32 (0.25) |
| 11 | 4.5 | 40 | 24.67 (0.02) | 76.01 | 0.47 (0.07) | 0.476 (0.06) | 30 (0.22) |
| 12 | 3.5 | 32 | 21.14 (0.01) | 83.25 | 0.29 (0.01) | 0.606 (0.08) | 25 (0.26) |
| 13 | 7.0 | 44 | 31.49 (0.04) | 85.23 | 0.56 (0.09) | 0.398 (0.03) | 34 (0.33) |
| Run | A (%) | B (%) | TSa (MPa) | DTb (min) | DTPb (min) | Pyc (MPa) | FESa (%) | Espa (J g−1) |
|---|---|---|---|---|---|---|---|---|
| a These values were determined under the compaction force of 176 MPa.b Tablets having a breaking force of 60 N were used for the determination.c The yield pressure was determined under the compaction force range of 50–110 MPa.d Standard deviation.e A, HPMC in the feed solid (%, w/w); B, the solid content of the feed (%, w/v). TS, tensile strength; DT, disintegration time of tablets containing the coprocessed excipient only; DTP, disintegration time of tablets composed of 96.5% the coprocessed excipient and 3.5% PVPP; Py, yield pressure; FES, fast elastic stretch; Esp, unit effective compaction work. | ||||||||
| 1 | 10.5 | 32 | 4.40 (0.04)d | 28.41 (0.02) | 17.04 (0.01) | 298.33 (0.21) | 8.16 (0.21) | 13.73 (0.19) |
| 2 | 7.0 | 32 | 3.33 (0.05) | 17.07 (0.03) | 9.70 (0.04) | 316.26 (0.18) | 8.97 (0.12) | 13.52 (0.12) |
| 3 | 4.5 | 23 | 1.85 (0.05) | 15.74 (0.05) | 5.65 (0.01) | 355.49 (0.17) | 8.79 (0.22) | 11.13 (0.23) |
| 4 | 9.5 | 23 | 4.09 (0.04) | 23.63 (0.09) | 15.73 (0.09) | 308.56 (0.22) | 8.08 (0.18) | 14.64 (0.27) |
| 5 | 7.0 | 19 | 2.98 (0.07) | 16.75 (0.02) | 10.88 (0.01) | 316.95 (0.29) | 8.49 (0.11) | 12.49 (0.21) |
| 6 | 7.0 | 32 | 3.28 (0.05) | 15.70 (0.02) | 9.90 (0.03) | 322.86 (0.11) | 8.73 (0.21) | 12.98 (0.18) |
| 7 | 7.0 | 32 | 3.32 (0.04) | 16.13 (0.01) | 9.61 (0.05) | 315.56 (0.16) | 8.68 (0.28) | 13.51 (0.16) |
| 8 | 7.0 | 32 | 3.35 (0.03) | 16.03 (0.04) | 9.66 (0.05) | 316.21 (0.21) | 8.87 (0.23) | 13.45 (0.24) |
| 9 | 9.5 | 40 | 4.13 (0.04) | 21.35 (0.03) | 14.91 (0.02) | 303.88 (0.18) | 8.18 (0.17) | 13.90 (0.16) |
| 10 | 7.0 | 32 | 3.31 (0.03) | 16.90 (0.08) | 9.31 (0.02) | 316.04 (0.24) | 8.84 (0.16) | 13.09 (0.13) |
| 11 | 4.5 | 40 | 2.35 (0.03) | 15.29 (0.12) | 5.18 (0.02) | 354.38 (0.23) | 8.39 (0.22) | 12.00 (0.21) |
| 12 | 3.5 | 32 | 1.59 (0.04) | 14.26 (0.06) | 4.34 (0.03) | 358.66 (0.19) | 8.56 (0.10) | 11.01 (0.25) |
| 13 | 7.0 | 44 | 3.23 (0.04) | 15.86 (0.08) | 8.71 (0.01) | 313.36 (0.31) | 8.81 (0.15) | 13.31 (0.17) |
| Responses | Model F-value | Prob > F | Lack of fit F-value | Prob > F | St. dev. | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|---|---|---|---|
| a DT, disintegration time of tablets containing the co-processed excipient only; DTP, disintegration time of tablets composed of 96.5% the co-processed excipient and 3.5% PVPP; Esp, unit effective compaction work. | ||||||||
| Angle of repose (°) | 41.50 | 0.0005 | 1.36 | 0.35 | 0.47 | 0.9765 | 0.9529 | 0.8370 |
| Median particle size (μm) | 248.97 | <0.0001 | 4.77 | 0.08 | 0.65 | 0.9920 | 0.9880 | 0.9687 |
| Bulk density (g cm−3) | 112.64 | <0.0001 | 4.95 | 0.07 | 0.02 | 0.9575 | 0.9490 | 0.8932 |
| Tensile strength (MPa) | 874.25 | <0.0001 | 5.30 | 0.07 | 0.04 | 0.9984 | 0.9973 | 0.9904 |
| DT (min) | 113.76 | <0.0001 | 0.48 | 0.65 | 0.54 | 0.9913 | 0.9826 | 0.9646 |
| DTP (min) | 476.18 | <0.0001 | 4.28 | 0.09 | 0.36 | 0.9937 | 0.9917 | 0.9812 |
| Yield pressure (MPa) | 97.39 | <0.0001 | 3.49 | 0.12 | 5.92 | 0.9512 | 0.9414 | 0.8710 |
| Fast elastic stretch (%) | 6.72 | 0.0113 | 3.60 | 0.12 | 0.17 | 0.7706 | 0.6559 | 0.1878 |
| Esp (J g−1) | 25.60 | 0.0001 | 2.77 | 0.17 | 0.35 | 0.9275 | 0.8913 | 0.7312 |
3.2. Combined effects of the solid content of the feed and % HPMC
The prediction equations of the developed response surface models in function of the amount of HPMC in the feed solid and the solid content of the feed suspension are mentioned in Table 7, while the corresponding contour plots and three-dimensional response surfaces plots generated by the regression models are shown in Fig. 1 and 2, which show the relationship between the independent variables and the responses graphically. Among the symbols used, A, B, A2, and B2 represent the single effect on the responses by the two independent variables (i.e., % HPMC in the feed solid and the solid content of the feed), with A and B referring to the interaction effects.| a A, HPMC in the feed solid (%, w/w); B, the solid content of the feed (%, w/v). DT, disintegration time of tablets containing the coprocessed excipient only; DTP, disintegration time of tablets composed of 96.5% the coprocessed excipient and 3.5% PVPP; Esp, unit effective compaction work. |
|---|
| Angle of repose = 31.80 + 2.83 × A + 0.48 × B + 0.75 × A × B − 1.34 × A2 + 0.66 × B2 − 2.08 × A × B2 |
| The median particle size = 29.43 + 6.81 × A + 2.18 × B + 1.05 × A × B + 0.64 × A2 |
| Bulk density = 0.37 − 0.095 × A + 0.039 × A2 |
| Tensile strength = 3.32 + 1.00 × A + 0.11 × B − 0.11 × A × B − 0.15 × A2 − 0.09 × B2 |
| DT = 16.37 + 5.00 × A − 0.50 × B − 0.46 × A × B + 2.53 × A2 + 0.02 × B2 − 1.52 × A × B2 |
| DTP = 9.71 + 4.72 × A − 0.54 × B + 0.54 × A2 |
| Yield pressure = 318.13 − 22.84 × A + 7.60 × A2 |
| Esp = 13.22 + 1.16 × A + 0.16 × B − 0.40 × A × B − 0.38 × A2 |
The results in Fig. 1a2 and 2b2 show that when the solid content of the feed was constant, the median particle size of the spray dried powders increased as the amount of HPMC increased. This is because that there is more and more HPMC available to agglomerate the suspended CC particles. In addition, a higher content of HPMC produced more viscous sprayed droplets, and then a layer of more completely thin film formed on the surface of composition particles, wrapping more primary CC particles inside. When the amount of HPMC was constant, the median particle size of the spray dried powders increased with increasing the solid content of the feed. This may be due to the larger volume occupied by the solid fraction, resulting in more particle collisions and agglomeration. In addition, the effect of the two factors on median particle size exhibited certain interaction (runs 1, 4, and 9) (Table 4).
The decrease in bulk density with increasing % HPMC was due to the low bulk density of HPMC. In addition, in the spray drying process, two raw materials were intimately and homogeneously combined to form the composite particles and the surfaces of the primary and composite particles were covered by HPMC macromolecules. Thus, the outer and inner surface properties of the composite particles become identical with those of the pure binder material.34 In contrast, the solid content of the feed had much less influence on the bulk density of the spray dried powders (run 5 vs. 6 and 3 vs. 11) (Table 4).
In this study, as shown in Table 5, two sets of disintegration time were determined, which corresponded to tablets containing 3.5% PVPP or not. The compacts, tableted with the co-processed excipient alone and having a breaking force of ∼60 N, exhibited a disintegration time of 14.26–28.41 min. Whereas, the disintegration time of most tablets with the same breaking force but containing 3.5% PVPP decreased to 4.34–14.91 min, less than the 15 min requirement for rapid release tablets in the European pharmacopoeia. Therefore, disintegration appears not to be a limiting factor for the application of the co-processed excipient developed here.
Tableting parameters, including yield pressure (Py), Esp, and fast elastic stretch, for the excipients studied were recorded and are summarized in Table 5. Py is a parameter related to the ability of a material to deform under pressure.35,36 Typically, lower values of Py indicate the onset of deformation at lower applied pressures. The results in Fig. 1a7 and 2b7 indicate that when the amount of HPMC was constant, the solid content of the feed had no effect on Py. When the solid content of the feed was constant, Py decreased as the amount of HPMC increased. For example, the powders prepared by runs 1 and 4 with a high content of HPMC (10.5% and 9.5% w/w, respectively) had a significantly lower Py than those with low amounts of HPMC (3.5 w/w for run 12 and 4.5 w/w for run 3) (Table 5). This is attributed to the plastic deformation nature of HPMC.
The Esp values, which represent the energy retained in the tablet after unloading and are related to the deformation properties of tested materials as well as their binding properties, were calculated based on the works recorded by the press. When the amount of HPMC was constant, Esp decreased as the solid content of the feed decreased. The lower the solid content is, the lower the HPMC concentration and the system viscosity are, and thus, the higher percentage of small atomized droplets and small dried particles are produced in order. During tableting, small particles tend to increase the friction, and thus, decrease the unit effective compaction work Esp. When the solid content of the feed was constant, Esp increased with HPMC level up to a maximum, beyond which the value decreased with further increases in HPMC levels (Fig. 1a8 and 2b8). In addition, the effect of the two factors on Esp exhibited certain interaction. As to fast elastic stretch, no clear trends were observed, suggesting that the effects of the two factors are both insignificant or are offset from each other.
3.3. Optimization and model validation
Tablet tensile strength and disintegration time are two key considerations for excipients used in tablets. Tensile strength is important for assessing the binding capacity and compactibility of the excipient. And, tablet disintegration time can affect the drug release rate, which is related to the drug absorption and efficacy. Therefore, maximum tensile strength and minimum DTP were both chosen as the optimization criteria in this study. Numerical optimization was performed using the Design-Expert software to find the optimal values of the independent variables. Finally, the optimized feed composition was achieved, i.e., the HPMC level in the feed solid: A = 6.7% (w/w) and the solid content of the feed: B = 42% (w/v). These values predict tablet tensile strength of 3.21 MPa and DTP of 8.48 min. An additional experiment was performed to verify the choice feed composition. The results are shown in Table 8. The low value of prediction error (not more than 6.24%) indicates that the observed responses are in close agreement with the predicted values and that the model is valid. It can also be concluded that the optimization technique is appropriate for optimizing the feed composition to produce the co-processed excipient of CC and HPMC by co-spray drying.| Responses | Observed values | Predicted values | Prediction errors (%) |
|---|---|---|---|
| a Standard deviation.b DT, disintegration time of tablets containing the coprocessed excipient only; DTP, disintegration time of tablets composed of 96.5% the coprocessed excipient and 3.5% PVPP; Esp, unit effective compaction work. | |||
| AR (°) | 34.86 (0.32)a | 33.32 | 4.62 |
| Bulk density (g mL−1) | 0.39 (0.03) | 0.39 | 0 |
| Median particle size (μm) | 31.94 (0.02) | 30.97 | 3.13 |
| Tensile strength (MPa) | 3.28 (0.02) | 3.21 | 2.18 |
| DT (min) | 16.52 (0.01) | 15.55 | 6.24 |
| DTP (min) | 8.91 (0.02) | 8.48 | 5.07 |
| Yield pressure (MPa) | 319.20 (0.34) | 321.03 | −0.57 |
| Esp (J g−1) | 13.69 (0.19) | 13.33 | 2.70 |
4. Conclusions
To develop a novel co-processed tablet excipient based on calcium carbonate and HPMC, the central composite design-response surface methodology (CCD-RSM) was applied in this study to optimize the amount of HPMC E3 and the solid content of the feed used for co-spray drying. The results show that the surface models developed for powder flowability, median particle size, bulk density, tablet tensile strength, disintegration time, yield pressure, Esp, and fast elastic stretch were significant, without significant lack of fit. However, modeling of production yield and moisture content was not significant. By variance and regression analysis, the quadratic regression equation was established as a predicted model. The optimal conditions were obtained at 6.7% (w/w) for the HPMC amount in the feed solid and 42% (w/v) for the solid content of the feed. The experimental values of the co-processed excipient prepared under the optimized conditions were mostly close to the model predicted values. Under these conditions, the tablet tensile strength and disintegration time were 3.28 MPa and 8.91 min, respectively. The combination use of CCD, RSM, and the desirability function can provide an insight into a lab-scale pharmaceutical study, and quickly obtain the optimal conditions, saving experimental time and materials as well as personal costs. It can provide theoretical guidance for further development of co-processed excipients based on calcium carbonate and HPMC.Conflict of interest
The authors report no conflicts of interest.Acknowledgements
This work was supported by the Innovation Program of Shanghai Municipal Education Commission (13ZZ098); the funds from Shanghai Municipal Commission of Health and Family Planning (ZY3-CCCX-3-5001) and Science and Technology Commission of Shanghai Municipality (15DZ2292000); the Science and Technology Development Fund of Pudong New Area (PKF-2013-003); and the natural science fund within budget of Shanghai university of traditional Chinese medicine (2013JW27).References
- P. Shivanand and O. L. Sprockel, Powder Technol., 1992, 69, 177–184 CrossRef CAS.
- S. K. Nachaegari and A. K. Bansal, Pharm. Technol., 2004, 28, 52–65 CAS.
- P. Gupta, S. K. Nachaegari and A. K. Bansal, in Excipient Development for Pharmaceutical: Biotechnology and Drug Delivery Systems, ed. A. Katdare and M. V. Chaubal, Informa Healthcare, USA, 2006, pp. 109–126 Search PubMed.
- J. Rojas, I. Buckner and V. Kumar, Drug Dev. Ind. Pharm., 2012, 38, 1159–1170 CrossRef CAS PubMed.
- S. M. Ambore, J. Tekale and S. G. Gattani, World Appl. Sci. J., 2014, 31, 801–810 CAS.
- C. Bacher, P. M. Olsen and P. Bertelsen, et al., Int. J. Pharm., 2007, 342, 115–123 CrossRef CAS PubMed.
- M. Delour desgarzonserra and L. Villafuerterobles, Int. J. Pharm., 2003, 258, 153–163 CrossRef.
- L. V. Allen, Int. J. Pharm. Compd., 2000, 4, 306–310 Search PubMed.
- R. C. Rowe, P. J. Sheskey and M. E. Quinn, Handbook of Pharmaceutical Excipients, the Pharmaceutical Press, London, UK, 6th edition, 2009, pp. 326–329 Search PubMed.
- R. J. Roberts and R. C. Rowe, J. Pharm. Pharmacol., 1985, 37, 377–384 CrossRef CAS PubMed.
- S. Mattsson and C. Nystrom, Eur. J. Pharm. Sci., 2000, 10, 53–66 CrossRef CAS PubMed.
- Y. Wang, Y. Xie and D. Xu, et al., Drying Technol., 2014, 32, 557–566 CrossRef CAS.
- Y. Gonnissen, E. Verhoeven and E. Peeters, et al., Eur. J. Pharm. Biopharm., 2008, 69, 320–334 CrossRef CAS PubMed.
- X. Lin, C. W. Chyi and K. F. Ruan, et al., Eur. J. Pharm. Biopharm., 2011, 79, 406–415 CrossRef CAS PubMed.
- C. Huang, H. Wu and R. F. Li, et al., New Biotechnol., 2012, 29, 372–378 CrossRef CAS PubMed.
- M. Dutta and K. B. Jayanta, Int. J. Environ. Sci. Technol., 2012, 5, 42–53 CrossRef CAS.
- M. Jain, V. K. Garg and K. Kadirvelu, Bioresour. Technol., 2011, 102, 600–605 CrossRef CAS PubMed.
- N. F. Rahmati, M. M. Tehrani and K. Daneshvar, Food Biophys., 2015, 10, 39–50 CrossRef.
- X. L. Liu, T. H. Mu and H. N. Sun, et al., Food Chem., 2013, 141, 3034–3041 CrossRef CAS PubMed.
- R. Chawla, S. Jaiswal and B. Mishra, Expert Opin. Drug Delivery, 2014, 11, 31–43 CrossRef CAS PubMed.
- Y. Y. Bei, X. F. Zhou and B. G. You, et al., Int. J. Nanomed., 2013, 8, 1795–1808 Search PubMed.
- J. Prakash Maran, V. Mekala and S. Manikandan, Carbohydr. Polym., 2013, 92, 2018–2026 CrossRef CAS PubMed.
- V. Samavati, Int. J. Biol. Macromol., 2013, 61, 142–149 CrossRef CAS PubMed.
- J. Prakash Maran, V. Sivakumar and R. Sridhar, Ind. Crops Prod., 2013, 42, 159–168 CrossRef CAS.
- J. T. Fell and J. M. Newton, J. Pharm. Sci., 1970, 59, 688–691 CrossRef CAS PubMed.
- P. J. Denny, Powder Technol., 2002, 127, 162–172 CrossRef CAS.
- N. A. Armstrong and H.-N. Rf, J. Pharm. Pharmacol., 1972, 24, 135P–136P CrossRef CAS PubMed.
- J. Li, F. Wu and X. Lin, et al., RSC Adv., 2015, 5, 69289–69298 RSC.
- Z. Ma, B. Gao and P. Wu, et al., RSC Adv., 2015, 5, 21042–21049 RSC.
- Q. Wang, J. Zhang and A. Wang, RSC Adv., 2013, 3, 23423–23431 RSC.
- G. D. Park and Y. Kang, RSC Adv., 2014, 4, 44203–44207 RSC.
- S. Wang, J. Li and X. Lin, et al., Int. J. Pharm., 2015, 486, 370–379 CrossRef CAS PubMed.
- K. Masters, SprayDryConsult Intl, ApS, Denmark, 2002 Search PubMed.
- S. Adolfsson, C. Caramella and C. Nystrom, Int. J. Pharm., 1998, 160, 187–195 CrossRef.
- P. Paronen, Drug Dev. Ind. Pharm., 1986, 12, 1903–1912 CrossRef CAS.
- M. Duberg and C. Nystrom, Powder Technol., 1986, 46, 67–75 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |


