Insights into the enhancement mechanism coupled with adapted adsorption behavior from mineralogical aspects in bioleaching of copper-bearing sulfide ore by Acidithiobacillus sp.†
Abstract
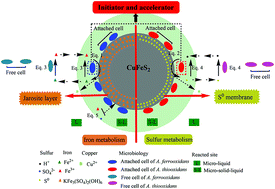
The enhancement mechanism of adapted adsorption behavior in the bioleaching of copper-bearing sulfide ore by Acidithiobacillus sp. was systematically investigated from a mineralogical viewpoint and compared to adsorption-deficient (DF) and adsorption-unadapted (UA) systems. With the assistance of the adapted adsorption behavior, both iron and sulfur metabolism was enhanced, which was proven by a series analysis of key chemical parameters, including scanning electron microscopy (SEM) and X-ray diffraction (XRD). SEM analysis revealed smaller jarosite and S0 granules as well as more potential adsorption sites on the ore’s surface, thus indicating a stronger “contact” mechanism. XRD analysis showed that more chemical derivatives were generated owing to active iron/sulfur metabolism. Additionally, the attached and free biomasses of A. ferrooxidans and A. thiooxidans were increased by 33.3–58.9% and 25.0–33.9%, respectively. Moreover, the final concentration of the extracted copper ions was improved by 22.8% (A. ferrooxidans) and 28.9% (A. thiooxidans). All results proved that the adsorption behavior coupled to the attached cells was greatly stimulated and accelerated by the adapted evolution and further contributed to a higher bioleaching efficiency. The adapted method and its mechanism will be useful to further guide similar bioleaching processes in the near future.

 Please wait while we load your content...
Please wait while we load your content...