4a,8a-Azaboranaphthalene-4-yl phosphine ligands: synthesis and electronic modulation in Suzuki–Miyaura coupling reactions†
Feiye Suna,
Min Huanga,
Zhaohui Zhoub and
Xiangdong Fang*a
aDepartment of Chemistry, Shanghai Key Laboratory of Chemical Assessment and Sustainability (CAS), Tongji University, 1239 Siping Road, Yangpu District, Shanghai 200092, People's Republic of China. E-mail: xdfang@tongji.edu.cn
bDepartment of Chemistry, Xiamen University, Xiamen, Fujian 361005, People's Republic of China
First published on 28th August 2015
Abstract
The preparation of a new class of triarylphosphine ligands consisting of the 4a,8a-azaboranapthalene-4-yl (ABN) group is disclosed. X-ray analysis and DFT calculations demonstrate a characteristic π-conjugation system of ABN-phosphine 3, which has been evaluated as a supporting ligand in Pd-catalysed Suzuki–Miyaura coupling reactions of aryl halides and phenyl boronic acid.
Transition-metal catalysed cross-coupling reactions are arguably one of the most efficient synthetic strategies that have been utilised in the preparation of aromatic or heteroaromatic compounds.1 To facilitate the above catalytic transformations, phosphines are generally used as the supporting ligand with excellent electronic and steric tunability, which thereof provides influential modulation to the catalytic metal centre.2 In particular, arylphosphine ligands are of great importance in homogeneous transition-metal catalysis due to their advantageous participation in metal-catalysed carbon–carbon and carbon-heteroatom bond-forming reactions.3 For example, bulky and electron-rich monodentate dialkylbiarylphosphines have been designed and utilised by Buchwald to promote the cross-coupling reactions of unactivated aryl chlorides;2b,4 while triarylphosphines have intriguing applications in asymmetric catalysis and small molecule activation.5 In fact, the catalytic property of the phosphine-ligated metal centre is directly related to the lone-pair electron density of phosphorus, which is intrinsically influenced by the nature of the P-attached groups.6
In line with our interest in BN-substituted aromatics, we recently reported a gram-scale synthesis of 4a,8a-azaboranaphthalene (ABN).7 The trifecta features of ABN molecules with their unique molecular framework (e.g. embedded BN Lewis pair), impressive thermodynamic stability and relatively straightforward synthesis encourages us to investigate a new class of phosphine ligands with ABN-surrogates to substitute traditional all-carbon aryl groups. In addition, we reasoned that the electron density at the C4/C5 as well as C2/C7 in the ABN ring of 1 should increase relatively as expected by the resonance structures of ABN molecule, suggesting more sp3-hybridised character at both carbons (1a and 1b in Fig. 1), which may result in more electron-donating ABN-phosphine ligands relative to the corresponding arylphosphines (Fig. 2).
As a merit outcome of excellent functional-group tolerance, late transition-metal (e.g. Pd/Cu/Ni) catalysed direct C–P(III) coupling reactions involving secondary phosphines and aryl halides have been developed as an important approach for the synthesis of arylphosphine compounds.8 However, to our best knowledge, metal-catalysed coupling synthesis of ABN-phosphine compounds has never been reported in the volume of literature and their function as supporting ligands in metal catalysis remains completely unexplored as well. Herein, we debut a copper-catalysed preparation of ABN-phosphine compounds that allows direct and efficient C–P(III) coupling of 4-iodo-ABN and diarylphosphine at 100 °C. Moreover, the resulting ABN-phosphine can be employed as supporting ligand in Pd-catalysed Suzuki–Miyaura coupling reactions and the catalytic activity of the catalyst system has been evaluated in a preliminary prospect.
Our foregoing experiments indicated that the Grignard-type activation of iodo-ABN 2 by magnesium was unexpectedly difficult,7 which thus excluded the conventional preparation of ABN-phosphines involving reactions of the corresponding ABN-Grignard reagents with phosphine halides. To resolve this synthetic conundrum, we have refocused our interest on the metal-catalysed C–P(III) cross-coupling reactions. To our delight, CuI is found to catalyse the aforementioned C–P(III) coupling reactions of 2 and diphenylphosphine in the presence of inorganic bases such as Cs2CO3 at 100 °C (Table 1). The 31P NMR analysis of the isolated crystalline product suggested the presence of two different species with distinctive 31P NMR chemical shift values (δ) at 32.8 and −7.6 ppm respectively, which could be easily separated by flash column chromatography. As anticipated, the less polar compound of the two with δ(31P) = −7.6 ppm was characterized by NMR, HRMS, and X-ray single crystal diffraction experiment as ABN-phosphine 3; while the other one has been identified as the corresponding phosphine oxide 4. The yield of 3 relative to 4 is optimised by screening various catalysts as well as bases. It is found that the best degree of conversion of 3 can be achieved with the catalyst system of CuI and Cs2CO3 (Entry 3), though accompanying phosphine oxide 4 still persists in the product mixture.
| Entry | Base | Catalyst | Yield of 3 (%) | Yield of 4 (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-iodo-ABN (0.30 mmol), catalyst (0.015 mol, 5 mol%), Ph2PH(0.36 mmol, 1.2 equiv.), base (0.60 mmol), toluene (1.5 mL), 100 °C, 12 h.b Isolated yields based on the average of at least two independent runs after chromatography. | ||||
| 1 | Cs2CO3 | CuCl | 0 | 0 |
| 2 | Cs2CO3 | CuBr | 27 | 19 |
| 3 | Cs2CO3 | CuI | 50 | 22 |
| 4 | K2CO3 | CuI | 45 | 25 |
| 5 | K3PO4 | CuI | 38 | 21 |
| 6 | NaOMe | CuI | 22 | Trace |
| 7 | NEt3 | CuI | 0 | 0 |
These experimental findings indicate that the above reaction condition is at stake and its optimisation to avoid unnecessary oxidation may potentially lead to the selective preparation of ABN-phosphine 3. Even with vigorous Schlenk technique to carefully eliminate oxygen in the reaction atmosphere, phosphine oxide 4 has been repeatedly isolated, albeit at a relatively lower content in the case of Cs2CO3. We speculated that the oxygenated bases might contribute to the formation of phosphine oxide 4, though this conjecture needs further substantiation in the future work (Scheme 1). In fact, H2O2 oxidation of the mixture converts 3 to 4, which can be subsequently deoxygenated by HSiCl3 reduction to refurnish 3 in an overall 36% yield.9
When switching to sterically demanding di(o-tolyl) phosphine, the coupling reaction proceeds apparently in a different manner with no detectable formation of the corresponding phosphine oxide by 31P NMR analysis (only one 31P signal at δ = −11.5 ppm in the 31P{1H} NMR spectrum, see the ESI†) and affords exclusively ABN-phosphine 5 in 50% yield, in agreement with 1H NMR and HRMS results (Scheme 2). We attribute this observation to the increased steric hindrance in the environment of phosphorous that retards further oxidation. Unfortunately, attempts to obtain appropriate single crystals of 5 for X-ray diffraction analysis were unsuccessful at the present stage. Nonetheless, this observation reaffirms our previous argument that the oxidation mechanism of 3 to 4 is perhaps a process under kinetic control.
The fact that the steric influence of the ligand system may be translated into the chemoselective preparation of 3 stimulated us to optimise the reaction condition by screening proper ligands for the ABN-involved Cu-catalyzed C–P(III) coupling reactions (Table 2). As a secondary diamine, DMEDA (L1) was unable to improve either the yield or selective formation of 3 with a product ratio of roughly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Entry 1). In contrast, increasing the steric hindrance of the diamine system in L2 helps to provide almost perfect selectivity for the coupling reaction with no observation of phosphine oxide 4 in the final product even with a relatively lower overall yield (Entry 2). 1,10-Phenanthroline(L3)-ligated Cu catalyst system seems to foster both better yield and selectivity of the reaction, suggesting that the diimine ligands may supply the key to the successful optimisation of the reaction. In this regard, the steric bulkiness of the diimine ligand in L4 indeed gives the best catalytic result of the coupling reaction (Entry 4); while a noticeable deteriorated selectivity of the reaction is recorded using less steric demanding diimine ligand L5, but without impact on the yield (Entry 5).
1 (Entry 1). In contrast, increasing the steric hindrance of the diamine system in L2 helps to provide almost perfect selectivity for the coupling reaction with no observation of phosphine oxide 4 in the final product even with a relatively lower overall yield (Entry 2). 1,10-Phenanthroline(L3)-ligated Cu catalyst system seems to foster both better yield and selectivity of the reaction, suggesting that the diimine ligands may supply the key to the successful optimisation of the reaction. In this regard, the steric bulkiness of the diimine ligand in L4 indeed gives the best catalytic result of the coupling reaction (Entry 4); while a noticeable deteriorated selectivity of the reaction is recorded using less steric demanding diimine ligand L5, but without impact on the yield (Entry 5).
| Entry | Ligand | Yield of 3 (%) | Yield of 4 (%) |
|---|---|---|---|
| a Reaction conditions: 4-iodo-ABN (0.30 mmol), CuI (0.015 mmol, 5 mol%), Ph2PH (0.36 mmol, 1.2 equiv.), ligand (0.03 mmol, 10 mol%), Cs2CO3 (0.60 mmol), toluene (1.5 mL), 100 °C, 12 h.b Isolated yields based on the average of at least two independent runs after chromatography. | |||
| 1 | L1 | 19 | 14 |
| 2 | L2 | 25 | 0 |
| 3 | L3 | 47 | 10 |
| 4 | L4 | 85 | 0 |
| 5 | L5 | 77 | 9 |
ABN-phosphines 3 and 5 are both completely air- and moisture-stable in the solid state; while in the solution phase, 3 turns to be considerably air-sensitive and 5 keeps its notable stability. Single crystals of 3 suitable for X-ray diffraction analysis are grown by slow evaporation of its hexane solution at rt. The X-ray crystal structure of 3 is shown in Fig. 3, showing the ABN ring perfectly planar with all metric parameter values consistent with those found in typical BN aromatics.10 Perhaps, the most striking conformational feature of 3 in the solid state is pyramidal phosphorous atom with lone pair of electrons orienting toward the inner bay area of ABN. In this respect, two bulky phenyl groups point away from the C11–H bond such that the steric constraint may be minimised. Interestingly, the lone pair of the phosphorous atom lies out of the ABN ring plane with a dihedral angle of ca. 52°, a reminiscent character of π-conjugation system. These structural features are similarly observed by Venkataraman in the single crystal structure of (1-naphthyl)diphenylphosphine (L7).8d
 | ||
| Fig. 3 ORTEP drawing of 3. Thermal ellipsoids are shown at 30% probability and H-atoms are omitted for clarity. CCDC deposition number: 1416758. Selected bond lengths (Å) and angles (deg): C3–C14 1.371(2), C14–C8 1.423(3), C8–C13 1.347(3), N1–C13 1.387(3), P1–C1 1.834(2), P1–C3 1.828(2), B1–N1 1.461(3), C2–P1–C3–B1 1.56(5), C1–P1–C3–B1 104.69(5). | ||
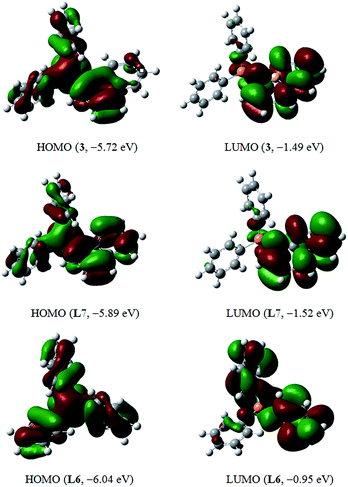
Density functional theory (DFT) studies at the RB3LYP/6-31++G(d,p) level are comparatively performed to provide some insightful glimpses of molecular electronic properties of PPh3 (L6), 3 and its corresponding carbon analogue (1-naphthyl)diphenylphosphine (L7) (Fig. 4 and the ESI†), indicative of completely different electronic structures resulting from the BN-substitution. First, both ABN- and naphthyl-phosphine compounds (3 and L7) possess narrowed HOMO–LUMO band gaps than PPh3 (5.09 eV), while 3 (4.24 eV) exhibits even smaller band gap than L7 (4.37 eV), consistent with our resonance structure argument and X-ray diffraction results. The calculated HOMOs of 3 and L7 have the largest molecular orbital coefficients at the phosphorous center, while the LUMOs of two ligands are dominated by the ABN and naphthyl groups respectively. In particular, the different HOMO energy levels of 3 and L7 contribute most to the relatively smaller band gap of 3, indicating more efficient orbital interaction between phosphorus and ABN in 3 for a better electron-density overlap.
The catalytic results of a Pd2(dba)3/phosphine catalyst system have been utilised to evaluate the activity of new phosphine ligand 3 in Suzuki–Miyaura coupling reactions of aryl halides and phenyl boronic acid (Table 3).11 All phosphine ligands (L6, L7, and 3) work efficiently with excellent yields in Pd-catalysed couplings of aryl bromide and PhB(OH)2 (Entries 1–3) under the reaction conditions described above. In contrast to PPh3 (L6) (Entry 6), ABN-phosphine 3 is relatively insensitive to steric congestion of the substrate with a similar coupling yield (Entry 13). Without the help of ligand, Pd2(dba)3 exhibits a reasonable activity in coupling of aryl bromide (Entry 4), but shows no sign of catalysing the coupling reactions of more difficult aryl chloride (Entry 5). This observation indicates that the Pd2(dba)3/phosphine catalyst system is well-suitable for gauging the ligand activity in the coupling reactions of aryl chlorides. In general, Pd-catalysed coupling reactions of electron-deficient aryl chlorides promoted by PPh3 afford less than 10% expected product (Entries 7 and 8) and no coupled product is detected in chlorobenzene and dichlorobenzene cases (Entries 9 and 10). In contrast, ABN-phosphine 3 clearly results in much improved yields of Pd-catalysed Suzuki couplings of the corresponding aryl chlorides (Entries 14–17). Finally, the carbon analogue (L7) of 3 gives apparently much less satisfied coupling results (Entries 11 and 12). The isosteric structures of 3 and L7 suggest that the electronic factors of two naphthalene ring structures differed by BN-substitution may be responsible for the catalytic disparity observed above. Further study of the coordination chemistry of BN-naphthyl phosphines may provide additional evidence for the experimental observations.
| Entry | Ar–X | Ligand | Product | Yield (%) |
|---|---|---|---|---|
| a Reaction conditions: aryl halides (0.5 mmol), phenyl boronic acid (1.5 equiv.), CsF (3 equiv.), Pd2(dba)3 (3 mol%), ligand (5 mol%), toluene (2 mL), 100 °C, 12 h.b Isolated yields based on the average of at least two independent runs after chromatography. | ||||
| 1 |  |
Ph3P (L6) |  |
88 |
| 2 |  |
 |
 |
92 |
| 3 |  |
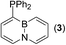 |
 |
92 |
| 4 |  |
N/A |  |
61 |
| 5 |  |
N/A |  |
0 |
| 6 |  |
L6 |  |
78 |
| 7 |  |
L6 |  |
8 |
| 8 |  |
L6 |  |
12 |
| 9 |  |
L6 |  |
0 |
| 10 |  |
L6 |  |
0 |
| 11 |  |
L7 |  |
21 |
| 12 |  |
L7 |  |
0 |
| 13 |  |
3 |  |
87 |
| 14 |  |
3 |  |
62 |
| 15 | 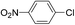 |
3 |  |
54 |
| 16 | 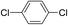 |
3 | 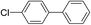 |
23 |
| 17 |  |
3 |  |
12 |
A mechanism involving the participation of a Pd(0) intermediate stabilised by ABN-phosphine ligand in the catalytic cycle is envisioned (Scheme 3). First, ABN-phosphine ligates to the Pd(0) species and extends the lifetime of the catalytic intermediate I by less dissociative coordination. Secondly, the Pd(0) metal centre with relatively increased d-electron density becomes more reactive toward the oxidative addition of C–Cl bond. Subsequent reductive elimination of Pd complex III regenerates the catalytic species I and liberates the cross-coupled product.
Conclusions
In summary, we have demonstrated for the first time that Cu-catalysed C–P(III) coupling reactions can be utilised to synthesise ABN-phosphine compounds 3 and 5. The electronic modulation of the catalyst system in Suzuki–Miyaura coupling reactions has been evaluated. The synthesis of ABN-phosphine ligands provides new arsenal to transition-metal catalysis, and the logical modification of the ABN-phosphine ligand system steering toward asymmetric catalysis and C–F bond activation is under investigation in our laboratory.Acknowledgements
We thank the Shanghai Pujiang Program (12PJ1408300) and the National Natural Science Foundation of China (21142006) for partial financial support of this project.Notes and references
- (a) J. Tsuji, Transition Metal Reagents and Catalysts: Innovations in Organic Synthesis, John Wiley & Sons, Chichester, 2000 Search PubMed; (b) J. F. Hartwig, Organotransition Metal Chemistry, from Bonding to Catalysis, University Science Books, New York, 2010 Search PubMed; (c) W. Shi, C. Liu and A. W. Lei, Chem. Soc. Rev., 2011, 40, 2761–2776 RSC; (d) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed; (e) C. C. C. J. Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CrossRef PubMed.
- (a) P. W. N. M. v. L. and P. C. J. Kamer, Phosphorus(III)Ligands in Homogeneous Catalysis: Design and Synthesis, John Wiley & Sons, Chichester, 2012 Search PubMed; (b) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS PubMed.
- (a) I. Ojima, Catalytic Asymmetric Synthesis, Wiley-VCH, Weiheim, 3rd edn, 2010 Search PubMed; (b) R. Noyori and T. Ohkuma, Angew. Chem., Int. Ed., 2001, 40, 40–73 CrossRef CAS; (c) B. Ghaffari, S. M. Preshlock, D. L. Plattner, R. J. Staples, P. E. Maligres, S. W. Krska, R. E. Maleczka and M. R. Smith, J. Am. Chem. Soc., 2014, 136, 14345–14348 CrossRef CAS PubMed.
- (a) A. F. Littke and G. C. Fu, Angew. Chem., Int. Ed., 2002, 41, 4176–4211 CrossRef CAS; (b) D. W. Old, J. P. Wolfe and S. L. Buchwald, J. Am. Chem. Soc., 1998, 120, 9722–9723 CrossRef CAS.
- (a) M. A. Courtemanche, M. A. Legare, L. Maron and F. G. Fontaine, J. Am. Chem. Soc., 2013, 135, 9326–9329 CrossRef CAS PubMed; (b) D. W. Stephan and G. Erker, Angew. Chem., Int. Ed., 2010, 49, 46–76 CrossRef CAS PubMed; (c) V. V. Grushin and W. J. Marshall, J. Am. Chem. Soc., 2004, 126, 3068–3069 CrossRef CAS PubMed; (d) Y. B. Yu, G. Z. He and X. G. Zhang, Angew. Chem., Int. Ed., 2014, 53, 10457–10461 CrossRef CAS PubMed.
- (a) J. P. Collman, J. R. Norton and R. G. Finke, Principles and Applications of Organotransition Metal Chemistry, University Science Books, Sausalito, 2nd edn, 1987 Search PubMed; (b) L. Noel-Duchesneau, N. Lugan, G. Lavigne, A. Labande and V. Cesar, Organometallics, 2014, 33, 5085–5088 CrossRef CAS.
- F. Y. Sun, L. L. Lv, M. Huang, Z. H. Zhou and X. D. Fang, Org. Lett., 2014, 16, 5024–5027 CrossRef CAS PubMed.
- (a) D. W. Cai, J. F. Payack, D. R. Bender, D. L. Hughes, T. R. Verhoeven and P. J. Reider, J. Org. Chem., 1994, 59, 7180–7181 CrossRef CAS; (b) O. Herd, A. Hessler, M. Hingst, M. Tepper and O. Stelzer, J. Organomet. Chem., 1996, 522, 69–76 CrossRef CAS; (c) A. Stadler and C. O. Kappe, Org. Lett., 2002, 4, 3541–3543 CrossRef CAS PubMed; (d) D. van Allen and D. Venkataraman, J. Org. Chem., 2003, 68, 4590–4593 CrossRef CAS PubMed; (e) D. Gelman, L. Jiang and S. L. Buchwald, Org. Lett., 2003, 5, 2315–2318 CrossRef CAS PubMed; (f) Y. L. Zhao, G. J. Wu, Y. Li, L. X. Gao and F. S. Han, Chem.–Eur. J., 2012, 18, 9622–9627 CrossRef CAS PubMed; (g) M. Kalek, A. Ziadi and J. Stawinski, Org. Lett., 2008, 10, 4637–4640 CrossRef CAS PubMed.
- Y. H. Li, L. Q. Lu, S. Das, S. Pisiewicz, K. Junge and M. Beller, J. Am. Chem. Soc., 2012, 134, 18325–18329 CrossRef CAS PubMed.
- (a) G. E. Herberich and H. Ohst, Adv. Organomet. Chem., 1986, 25, 199 CrossRef CAS; (b) A. J. Ashe III, Organometallics, 2009, 28, 4236 CrossRef.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685–4696 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1416758. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra15929f |
| This journal is © The Royal Society of Chemistry 2015 |