Mild Cu(OAc)2·H2O-catalyzed synthesis of multi-substituted 1,2,4-triazoles from amidines with nitriles via a N–N/C–N coupling†
Abstract
A simple and efficient Cu(OAc)2·H2O-catalyzed aerobic oxidation of amidines with nitriles for the synthesis of multi-substituted 1,2,4-triazoles has been achieved. The procedure constructs multi-substituted 1,2,4-triazoles and has the advantages of operational simplicity, broad substrate scope, and no need for prefunctionalized reagents. A possible mechanism has been proposed via the cascade N–H functionalization and N–N/C–N bond formation.
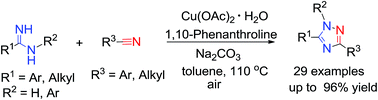

 Please wait while we load your content...
Please wait while we load your content...