Synthesis of a gemini quaternary ammonium salt and its reaction with wool fabric using click chemistry
Weicheng
Tian
b,
Yi
Hu
ab,
Wei
Wang
ab and
Dan
Yu
*abc
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai 201620, China. E-mail: yudan@dhu.edu.cn; Fax: +86-21-67792608; Tel: +86-21-67792456
bCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai 201620, China
cSaintyear Holding Group Co., Ltd, China
First published on 14th October 2015
Abstract
In this study, we have successfully synthesized a gemini quaternary ammonium salt C24H38O4N2Br2 and applied it to wool fabric to obtain antibacterial properties. First, tris(2-carboxyethyl)phosphine (TCEP) was utilized as a reducing agent to generate thiol groups on the surface of the wool fabric. Then, these thiol groups reacted with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of the gemini quaternary ammonium salt via click chemistry. The structure of the as-prepared ionic dimethacrylate (IDMA) monomers was characterized using FT-IR spectroscopy, 1H-NMR analysis, mass spectrometry and elemental analysis. The modified wool fabric exhibited good anti-bacterial properties against both E. coli and S. aureus. Furthermore, the modified fabric exhibits good antistatic properties and its mechanical properties are improved by the chemical bonds of the modification.
C groups of the gemini quaternary ammonium salt via click chemistry. The structure of the as-prepared ionic dimethacrylate (IDMA) monomers was characterized using FT-IR spectroscopy, 1H-NMR analysis, mass spectrometry and elemental analysis. The modified wool fabric exhibited good anti-bacterial properties against both E. coli and S. aureus. Furthermore, the modified fabric exhibits good antistatic properties and its mechanical properties are improved by the chemical bonds of the modification.
1. Introduction
Wool fibers are known as an important natural textile material because of their lightness, warmness, softness and smoothness.1 Their protein composition is analogous to the human skin surface and therefore they are potential materials for biological applications. However, wool products, other than fabrics, like carpets also act as a host for microorganism propagation, and are the nutrient source of worms and bacteria. Hence, having an antibacterial finishing on wool is gaining increasing attention.The general methods for preparing antibacterial fibers involve adsorbing, grafting or depositing some antibacterial materials. Hassan used plasma treatment prior to bonding with quaternary ammonium salts (QASs) to enhance the antibacterial properties of wool.2 Meanwhile, a variety of metals and their oxides have been explored for antimicrobial finishing of textiles, such as silver, metal complexes and quaternary ammonium groups on the fibers’ surface3 and other special compounds like chitosan.4–7 According to Qureshi’s research,8 the increased antimicrobial efficacy is attributed to the small particle size, which provides a large specific surface area, leading to greater interaction with micro-organisms. But the antibacterial properties will be gradually weakened by release of the antibacterial agent. Meanwhile, a major limitation of metal finishes may be the cause of environmental problems from the leaching out of heavy metals. Among the organic agents, QASs are well known as important biocides and have been used for many years.9 Numerous studies have demonstrated that the bioactivities of these agents depend upon the type of substituent, number of quaternary nitrogen atoms and the counter ions. It has been indicated that gemini QASs demonstrate a higher antibacterial potency than the corresponding mono-QASs.10 Furthermore, some studies have claimed that the antibacterial potencies of gemini QASs show a wider and more effective antimicrobial spectrum than those of mono-QASs against both Gram-negative and positive bacteria and fungi.11 There is no doubt that QASs are valuable in their application due to their excellent antimicrobial properties. However, they always show poor washing fastness because they are usually applied by absorbing or a pad-dry-cure process. A lack of chemical bonding makes them easy to leach out from the fibers. Taking the aforementioned into consideration, to improve the durability of antimicrobial finishes on wool, we have synthesized a gemini QAS and attempted to covalently attach it to the fibers in this study. Incorporating a high quantity of quaternary ammonium groups could significantly affect the overall surface properties, other than the antibacterial properties, as QASs as cationic agents can impart textiles with antistatic properties.
It is known that there are S–S groups in cystine and the cystine of wool protein molecules, which can be reduced to S–H groups by reducing agents, such as tris(2-carboxyethyl)phosphine hydrochloride (TCEP).12,13 These S–H groups could react with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds through a click-chemistry reaction under mild conditions, which is known as thiol–ene Michael addition.14 By this method, the wool fabric will be imparted with durable antibacterial properties and antistatic properties. Hence, we firstly synthesized a gemini QAS with C
C bonds through a click-chemistry reaction under mild conditions, which is known as thiol–ene Michael addition.14 By this method, the wool fabric will be imparted with durable antibacterial properties and antistatic properties. Hence, we firstly synthesized a gemini QAS with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups using the Menschutkin reaction, which is the addition reaction of tertiary amines with organo-halides.15 Then, we utilized TCEP to produce thiol groups on the wool fiber by cleavage of disulfide bonds. Finally, the as-prepared gemini QAS was grafted onto wool fibers through chemical bonding by thiol–ene click chemistry. The respective characterization studies were conducted in detail.
C groups using the Menschutkin reaction, which is the addition reaction of tertiary amines with organo-halides.15 Then, we utilized TCEP to produce thiol groups on the wool fiber by cleavage of disulfide bonds. Finally, the as-prepared gemini QAS was grafted onto wool fibers through chemical bonding by thiol–ene click chemistry. The respective characterization studies were conducted in detail.
2. Experimental
2.1. Materials
The woven wool fabric is a plain-weave 100% (Merino) of 190 g m−2 fabric mass, with a warp and weft yarn density of 23 yarns per cm and 20 yarns per cm in this test. 2-(Dimethylamino)ethyl methacrylate (DMAEMA), α,α′-dibromo-p-xylene and TCEP were purchased from Sigma Aldrich. Acetone, ethanol, sodium carbonate and sodium bicarbonate were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals were analytical grade reagents, and used without further purification.2.2. Synthesis of the gemini QAS (IDMA)
The reaction conditions to prepare IDMA were as follows: the ratio of DMAEMA and BbmBP was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the solvent was acetone, the temperature was 50 °C, and the reaction time was 24 h. After the reaction, the product was washed with acetone and carbon tetrachloride before suction filtration. The reaction yield was calculated using the actual value vs. the theoretical value. The actual value represents the weight of product after purifying and drying. The theoretical value represents the weight of the original chemical reagent inventory. The product was dried at 50 °C in a vacuum drying oven with yield of 94.6% obtained. The synthesis reaction equation is shown in Fig. 1.
1, the solvent was acetone, the temperature was 50 °C, and the reaction time was 24 h. After the reaction, the product was washed with acetone and carbon tetrachloride before suction filtration. The reaction yield was calculated using the actual value vs. the theoretical value. The actual value represents the weight of product after purifying and drying. The theoretical value represents the weight of the original chemical reagent inventory. The product was dried at 50 °C in a vacuum drying oven with yield of 94.6% obtained. The synthesis reaction equation is shown in Fig. 1.
2.3. Application of IDMA to the wool fabric
The wool fabric was pre-prepared in a water/ethanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution containing 20 mmol L−1 TCEP at pH 7.0 for 4 h at 25 °C according to our previous studies.16 The reaction equation is shown in Fig. 2. The processed wool fabric was added into a 5% IDMA solution at pH 8.0 for 8 h at 25 °C with a trace of TCEP as the catalyst. After treatment, the fabric was rinsed and dried to get the final product. The reaction equation is shown in Fig. 3.
1) solution containing 20 mmol L−1 TCEP at pH 7.0 for 4 h at 25 °C according to our previous studies.16 The reaction equation is shown in Fig. 2. The processed wool fabric was added into a 5% IDMA solution at pH 8.0 for 8 h at 25 °C with a trace of TCEP as the catalyst. After treatment, the fabric was rinsed and dried to get the final product. The reaction equation is shown in Fig. 3.
2.4. Characterization
FT-IR spectra (Nicolet 6700, Thermo Fisher, America), 1H-NMR analysis (Bruker AM-400, Avance 400, Switzerland), and mass spectrometry (Varian 310, Varian, America) were used to characterize the structure of the synthetic product. The surface functional groups were investigated using Fourier transform infrared analysis (Nicolet 6700, Thermo Fisher, America) and Raman analysis (InVia, Renishaw, Britain). The surface morphology of the fiber was observed using scanning electron microscopy (SEM, TM-1000, Hitachi). The antibacterial activity of the TCEP–IDMA treated wool fabric was tested against the Gram-negative bacteria E. coli and the Gram-positive bacteria S. aureus. The qualitative evaluation was performed according to the standard AATCC100-2004. The antistatic functionality was evaluated using a fabric induction electrostatic tester (YG(B)342E, Darong, China). The strength test was conducted according to the American standard (5035-95, 25 mm ± 2 mm, 75 mm ± 1 mm, 300 mm ± 10 mm min−1).3. Results
To analyze the chemical structure of the synthetic product, FT-IR spectroscopy, 1H-NMR analysis, mass spectrometry and elemental analysis were used. Curves a–c in Fig. 4 are the FT-IR spectra of DMAEMA, BbmBP and IDMA. The peak at 2772 cm−1 is lost but a new peak appears at 870 cm−1 in curve c when compared with curve a. These peaks are the characteristic absorption peaks of C–H groups in N(CH)3 and p-substituted benzene rings, respectively. The peak at 609 cm−1 is lost but new peaks appear at 1723 cm−1, 1293 cm−1 and 1170 cm−1 in curve c when compared with curve b, which are the characteristic absorption peaks of C–Br, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–C and C–N, respectively. In particular, the characteristic absorption peak of quaternary ammonium ions appears at 951 cm−1 in curve c. The FT-IR spectra show that the functional groups of the synthesis product correspond with the target QAS.
O, C–O–C and C–N, respectively. In particular, the characteristic absorption peak of quaternary ammonium ions appears at 951 cm−1 in curve c. The FT-IR spectra show that the functional groups of the synthesis product correspond with the target QAS.
The 1H-NMR analysis of the synthesis product IDMA is listed as follows: 1H-NMR (300 MHz, D2O): δ (ppm) 1.83 (s, 3H), 3.06 (s, 6H), 3.71 (t, 2H), 4.57 (s, 2H), 4.68 (t, 2H), 5.63 (s, 1H), 6.15 (s, 1H), 7.59 (m, 4H). All the expected H atoms have been found in the spectra without any impurity peaks. This indicates that the target product has been obtained with high purity. Mass spectrometry was used to confirm the relative molecular mass of IDMA. The mass spectra details of the synthesis product are as follows: the m/z peaks at 499.3 and 497.2 are the ion peaks of [M − Br−]+, and the m/z peak at 418.8 is the ion peak of [M − 2Br−]+. Thus, the target product was successfully synthesized with a molar mass of 578.4 g mol−1.
Raman spectra were used to characterize the effect of TCEP treatment on the wool fabric. Curves a and b in Fig. 5 are the Raman spectra of the untreated wool fabric and TCEP treated wool fabric. The peak at 522 cm−1 belongs to a weaker S–S peak in curve b, which indicates that the quantity of S–S became less through TCEP treatment. A new peak was found at 2575 cm−1 in curve b, which was attributed to a S–H vibration band. Thus, some S–S groups on the wool protein molecules were reduced to S–H groups after being treated with TCEP in the pretreatment.
The surface morphologies of the wool fibers are shown in Fig. 6, where (a)–(c) are the untreated wool fibers, TCEP pretreated wool fibers and TCEP–IDMA treated wool fibers. The wool cuticle layer and edge were clearly observed from Fig. 6(a). Compared with Fig. 6(a), they became obscure after TCEP pretreatment as shown in Fig. 6(b). For Fig. 6(c), there was not much difference observed which indicates that the surface morphology of the TCEP–IDMA treated wool fibers was similar to the untreated wool fibers. As seen from the SEM micrographs, it was indicated that the TCEP cleavage reaction and thiol–ene click reaction will not affect the wool fibers and the reactions are mild as we expected.
 | ||
| Fig. 6 SEM micrographs of the original wool fibers (a), the TCEP pretreated wool fibers (b) and the TCEP–IDMA treated wool fibers (c). | ||
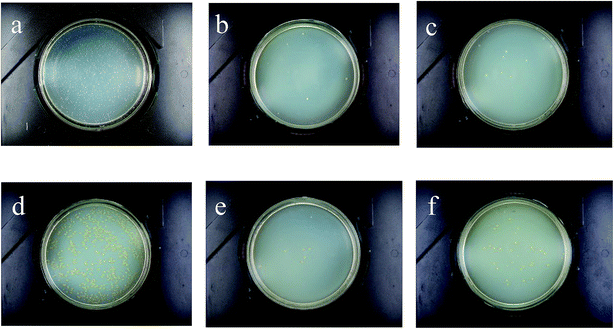
The antibacterial properties of the TCEP–IDMA treated wool fabric were measured according to the standard: AATCC100-2004. Fig. 7(a)–(f) show the results for E. coli and S. aureus on culture dishes which were contact-cultured with the original wool fabric, the TCEP–IDMA treated wool fabric and the treated wool fabric after 5 cycles of standard washing. In the experiment, “0” colonies of the test organism were recovered from the uninoculated treated wool test specimen swatches and a significant increase occurred in the number of bacteria recovered from the inoculated untreated wool control specimen swatches incubated for the specified contact time over the number of bacteria recovered from the inoculated untreated wool specimen swatches at “0” contact time, which means the bacteria grew normally and the experimental data were reliable. The antibacterial efficiency of the TCEP–IDMA treated wool fabric against E. coli is 94.2%. For the TCEP–IDMA treated wool fabric after 5 cycles of standard washing, only a few colonies were found indicating the antibacterial efficiency goes down to 86.3% against E. coli. The antibacterial efficiency of the TCEP–IDMA treated wool fabric against S. aureus is 90.1%. For the TCEP–IDMA treated wool fabric after 5 cycles of standard washing, only a few colonies were found indicating the antibacterial efficiency goes down to 83.9% against S. aureus. Compared with other literature values for similar materials, our result is not bad. For example, Liang et al.17 claimed the bacterial resistance rates of their product are all beyond 90%. This is close to the results of our observations and experiments. The conducted tests illustrate that the TCEP–IDMA treated wool fabric has a good and durable antibacterial ability. This is attributed to the strong bonding between the QAS and wool fabric from click chemistry.
The results of static voltage and half-life period of stored charge tests on the wool fabric are shown in Table 1, which represent the antistatic properties of the different fabrics. The half-life period of stored charge for the original wool fabric is 4.27 s. This indicates that the wool fabric has certain hydrophobic properties, so will easily accumulate static charge when being worn. The static voltage and half-life period of stored charge for the wool fabric both decreased by different degrees after TCEP treatment and TCEP–IDMA treatment. The results illustrate that the antistatic properties of the wool fabric were enhanced after treatment.
| Samples | Static voltage/V | The half-life period (s) |
|---|---|---|
| Original wool fabric | 1180 | 4.27 |
| TCEP treated wool fabric | 972 | 3.15 |
| TCEP–IDMA treated wool fabric | 494 | 1.08 |
In order to analyse the mechanical properties of the treated wool fabric, the break strength of the different wool fabrics was tested and the results are shown in Table 2. The breaking strength-MD (warp direction) of the original wool fabric is 234.0 N. A slight loss of the breaking strength was observed after TCEP pretreatment, which was due to the cleavage effect of TCEP as a mild reducing agent. It should be noted that the strength increased to 255.6 N along the warp direction after TCEP–IDMA treatment. The breaking strength-CMD along the weft direction had the same trend. The test results have been confirmed by repeating the test for 10 cycles. We attributed the reason to the crosslinking effect of the gemini QAS with the thiol groups on the wool fiber leading to the increased breaking strength after TCEP–IDMA treatment.
| Samples | Breaking strength MD (N) | Breaking strength CMD (N) |
|---|---|---|
| Original wool fabric | 234.0 | 125.5 |
| TCEP pretreated wool fabric | 227.0 | 118.6 |
| TECP–IDMA treated wool fabric | 255.6 | 126.7 |
4. Conclusions
A novel gemini quaternary ammonium salt IDMA was synthesized and reacted with wool fabric using click chemistry. The wool fabric was firstly treated with TCEP to produce thiol groups and then reacted with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of IDMA using click chemistry. The Raman spectra indicated that some S–S groups on the wool protein molecules were reduced to S–H groups after treatment with TCEP. From the SEM images, we could see the changes that occurred on the wool’s surface. The antibacterial efficiencies of the IDMA treated wool fabric and that fabric after 5 cycles of washing were 94.2% and 86.3% against E. coli. The antibacterial efficiencies of the IDMA treated wool fabric and that fabric after 5 cycles of washing were 90.1% and 83.9% against S. aureus. The static voltage and half-life period of electrostatic pressure for the wool fabric both reduced after the modification. The breaking strength tests indicated that the strength of the wool fabric was increased after treatment due to the crosslinking effect of the gemini QAS. In view of these results, this method provides an effective way to modify wool fabric with durable antibacterial and antistatic properties.
C groups of IDMA using click chemistry. The Raman spectra indicated that some S–S groups on the wool protein molecules were reduced to S–H groups after treatment with TCEP. From the SEM images, we could see the changes that occurred on the wool’s surface. The antibacterial efficiencies of the IDMA treated wool fabric and that fabric after 5 cycles of washing were 94.2% and 86.3% against E. coli. The antibacterial efficiencies of the IDMA treated wool fabric and that fabric after 5 cycles of washing were 90.1% and 83.9% against S. aureus. The static voltage and half-life period of electrostatic pressure for the wool fabric both reduced after the modification. The breaking strength tests indicated that the strength of the wool fabric was increased after treatment due to the crosslinking effect of the gemini QAS. In view of these results, this method provides an effective way to modify wool fabric with durable antibacterial and antistatic properties.
Acknowledgements
The research was supported by the National Natural Science Foundation of China (No. 51403032). The project was funded by the State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University.References
- M. Montazer and E. Pakdel, J. Text. Inst., 2011, 102, 343–352 CrossRef CAS PubMed.
- M. M. Hassan, Ind. Eng. Chem. Res., 2014, 53, 10954–10964 CrossRef CAS.
- B. Tomšič, B. Simončič, B. Orel, M. Žerjav, H. Schroers, A. Simončič and Z. Samardžija, Carbohydr. Polym., 2009, 75, 618–626 CrossRef PubMed.
- M. M. G. Fouda, R. Wittke, D. Knittel and E. Schollmeyer, Int. J. Diabetes Mellitus, 2009, 1, 61–64 CrossRef PubMed.
- S. Chernousova and M. Epple, Angew. Chem., Int. Ed. Engl., 2013, 52, 1636–1653 CrossRef CAS PubMed.
- Y. Liu, K. Ma, R. Li, X. Ren and T. S. Huang, Cellulose, 2013, 20, 3123–3130 CrossRef CAS.
- G. M. Raghavendra, T. Jayaramudu, K. Varaprasad, R. Sadiku, S. S. Ray and K. Mohana Raju, Carbohydr. Polym., 2013, 93, 553–560 CrossRef CAS PubMed.
- A. M. Qureshi, M. Qadir, A. Rauf, M. Idrees, S. Mumtaz, M. Najam-Ul-Haq, M. Ismail, M. Athar, R. Khushal and S. Riaz, Lett. Drug Des. Discovery, 2011, 8, 980–987 CrossRef CAS.
- S. Pavlukhina, Biomacromolecules, 2010, 11, 3448–3456 CrossRef CAS PubMed.
- Y. Zhang, M. Ding, L. Zhou, H. Tan, J. Li, H. Xiao, J. Li and J. Snow, Polym. Chem., 2012, 3, 907 RSC.
- A. Shirai, T. Maeda, H. Nagamune, H. Matsuki, S. Kaneshina and H. Kourai, Eur. J. Med. Chem., 2005, 40, 113–123 CrossRef CAS PubMed.
- P. Liu, B. W. O’Mara, B. M. Warrack, W. Wu, Y. Huang, Y. Zhang, R. Zhao, M. Lin, M. S. Ackerman and P. K. Hocknell, J. Am. Soc. Mass Spectrom., 2010, 21, 837–844 CrossRef CAS PubMed.
- J. A. Burns, J. C. Butler, J. Moran and G. M. Whitesides, J. Org. Chem., 1991, 22, 2648–2650 CrossRef.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Chem. Soc. Rev., 2010, 39, 1355–1387 RSC.
- J. M. Antonucci, D. N. Zeiger, K. Tang, S. Lin-Gibson, B. O. Fowler and N. J. Lin, Dent. Mater., 2012, 28, 219–228 CrossRef CAS PubMed.
- Y. Dan, J. Y. Cai, J. S. Church and L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 1236–1242 Search PubMed.
- Y. L. Liang, S. Y. Sui, P. Zhu, J. B. Zhang and Z. H. Dong, Wool Text. J., 2009, 37, 10–14 CAS.
| This journal is © The Royal Society of Chemistry 2015 |