High temperature dielectric relaxation anomalies in Ca0.9Nd0.1Ti0.9Al0.1O3−δ single crystals
Abstract
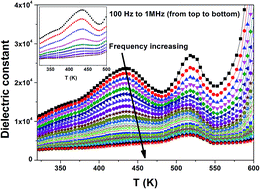
We herein report dielectric studies on Ca0.9Nd0.1Ti0.9Al0.1O3−δ single crystals grown by the optical floating zone technique in the temperature range from room temperature to 660 K. Two dielectric anomalies were observed at ∼436 K and 520 K, which correspond to relaxor-like behaviour due to Maxwell–Wagner relaxations and conduction relaxation associated with doubly ionized oxygen vacancies respectively. The presence of Ti3+ for charge carrier hopping and oxygen vacancies is confirmed by X-ray photoelectron spectroscopy analysis.

 Please wait while we load your content...
Please wait while we load your content...