Biodegradable containers composed of anionic liposomes and cationic polypeptide vesicles†
Abstract
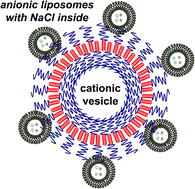
An electrostatic complexation of liposomes, composed of anionic palmitoyloleoyl phosphatidylserine (POPS1−) and zwitterionic dioleoylphophatidylcholine (DOPC), with bilayer vesicles composed of cationic poly(L-lysine)-b-poly(L-leucine) block copolypeptides has been investigated. The complexation was characterized by several physicochemical methods with the following main conclusions: (a) all added liposomes are totally adsorbed on the polypeptide vesicles up to a certain saturation concentration. (b) The calculated number of liposomes per a single polypeptide vesicle is about 60. (c) The liposomes remain intact (i.e., do not leak) after being complexed with the vesicles. (d) Complexes are stable in physiological ionic strength solution with [NaCl] = 0.15 M. (e) The vesicles are effectively digested by proteolytic enzyme trypsin even when covered by liposomes. These findings as well as the high potential for loading of anionic liposomes and cationic vesicles with biologically active compounds make these multi-liposomal complexes promising in the drug delivery field.

 Please wait while we load your content...
Please wait while we load your content...