Pd/C-catalyzed facile synthesis of primary aromatic amides by aminocarbonylation of aryl iodides using ammonia surrogates†
Abstract
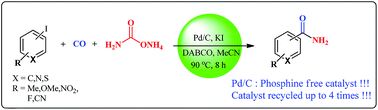
Use of Pd/C as an efficient, phosphine free, heterogeneous and recyclable catalyst for one pot carbonylative synthesis of primary aromatic amides has been demonstrated. The developed protocol is simple to perform and employs ammonium carbamate as an in situ solid ammonia source which is feasible and more convenient than direct use of ammonia gas. The applicability of the developed protocol was studied for various aryl iodide substrates and it was found that it provides good to excellent yields of the desired amides. The catalyst is easily separable and shows significant recyclability for up to four consecutive cycles without loss of catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...