Investigation of fate and behavior of tetracycline in nitrifying sludge system†
Abstract
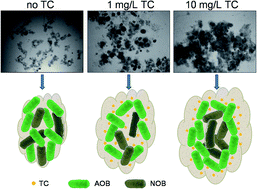
This study aims to investigate the fate and behavior of tetracycline (TC) in nitrifying sludge system, as well as the effects of TC dosage on sludge performance. For this purpose, two TC spiked and two control laboratory reactors were operated for two months, while the spiked reactors (designated as RI and RII) were intermittently fed with TC at the concentrations of 10 and 1 mg L−1, respectively. TC could be effectively removed via initial adsorption and subsequent biodegradation, while biodegradation was the primary mechanism in this study. Compared to RII, no significant negative effects were found on dehydrogenase activity under higher TC stress in RI. It is interesting that RI showed better nitrification performance than RII, especially higher nitrite oxidation capacity. Moreover, exposure to TC also promoted the formation of aggregation and affected the composition of nitrifying bacteria. The relative contents of nitrite-oxidizing bacteria (NOB) in RII decreased by almost 50%, from 11.4 ± 3.2% to 6.5 ± 2.5% while there was a slight change in RI, from 11.9 ± 5.8% to 11.2 ± 3.8%. Furthermore, the mean sludge diameter increased from 218.3 ± 7.8 μm to 512.4 ± 7.8 μm and 353.8 ± 11.1 μm in RI and RII, respectively. This indicated that larger aggregations were discovered in reactors with high TC stress. The aggregation might lead to a multilayer structure of sludge to protect the microorganism inside, which would explain the higher relative abundance of NOB in reactors with high TC stress. This work expands our vision about the fate and behavior of antibiotics in activated sludge system, which has far-reaching implications in activated sludge processes.

 Please wait while we load your content...
Please wait while we load your content...