The new dicyanoruthenium(III) building block with 2′-hydroxyacetophenone imine for heterobimetallic complexes†
Jing Rua,
Tian-Xiang Zonga,
Jing-Yuan Gea,
Min-Xia Yaob and
Jing-Lin Zuo*a
aState Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Collaborative Innovation Center of Advanced Microstructures, Nanjing University, Nanjing 210093, P. R. China. E-mail: zuojl@nju.edu.cn; Fax: +86-25-83314502
bSchool of Science, Nanjing University of Technology, Nanjing 210009, P. R. China
First published on 23rd October 2015
Abstract
A new ruthenium(III)-based building block, trans-(Ph4P)[RuIII(L)2(CN)2] (L = 2′-hydroxyacetophenone imine), has been synthesized and characterized. Reactions of this building block with different MnIII Schiff base (SB) complexes, [Mn(SB)(H2O)2]ClO4, result in 1-D zigzag chain complexes, [RuIII(L)2(CN)2MnIII(SB)]n (SB = salen, 1; salcy, 2; nappa, 4; napcy, 5), respectively. X-ray crystallographic studies reveal that each of the MnIII centers has a distorted octahedral environment, while complex 3 possesses a single cationic chain structure consisting of [Cu(chxn)2][Ru(L)2(CN)2]n units and their corresponding [Ru(L)2(CN)2]− anions. Compounds 1–3 exhibit antiferromagnetic coupling between the RuIII and MnIII/CuII centers, whereas 4 and 5 reveal ferromagnetic coupling between the RuIII and MnIII centers through the cyano bridges. Furthermore, magneto-structural correlation for some typical cyano-bridged heterobimetallic RuIII–MnIII/CuII compounds is discussed.
Introduction
Low-dimensional magnetic materials, such as single-molecule magnets (SMMs) and single-chain magnets (SCMs) with slow magnetic relaxation, have been extensively studied due to their unique potential applications for molecular devices, high-density information storage, and quantum computers.1Recently, more and more molecule-based magnets based on 4d and 5d metal ions have been reported.2 An essential driving force for the interest in this area is because the heavier metal ions can contribute significantly to the physical properties of paramagnetic compounds. More specifically, the heavier metals possess more diffuse valent orbitals than 3d metals, which can result in stronger magnetic exchange interactions.3 The 4d/5d metal ions are characterized by large spin–orbit coupling that is instrumental in magnetic anisotropy.4,5 In the synthesis of paramagnetic materials, most efforts have been devoted to cyanide precursors, especially to those of Nb,6 Mo,7 W,8 and Re9 compounds, whereas low-dimensional magnetic materials incorporating Ru and Os units received less attention. With regard to the Ru(III)-containing building blocks, such like trans-[RuIII(L1)2(CN)2]− (L1 = 8-quinolinolato (Q) and acetylacetone anion (acac))10 and trans-[RuIII(salen)(CN)2]− (H2salen = N,N′-bis(salicylidene)ethylenediamine),11 they are used to construct a series of RuIII-3d and RuIII-4f polynuclear complexes that exhibit a variety of novel structural and magnetic properties. Recently, a new heteroleptic cyanido-Schiff base complex [K(H2O)2RuIII(valen)(CN)2]·H2O as a building block and its corresponding RuIII-4f heterometallic complexes with one-dimensional structures have been reported.12
Given the above mentioned trans-dicyano Ru complexes as useful building blocks, low-dimensional magnetic materials are easily constructed via the cyano bridges.13 For example, when building blocks of ((R,R) or (S,S))-[Bu4N][Ru(5-Cl-salcy)](CN)2] (salcy = N,N′-(1,2-cyclohexanediylethylene)bis(salicylideneiminato)dianion) and [Ru(salen)(CN)2]− are employed, a series of cyano-bridged chains and clusters that behave as ferrimagnets, SCMs, or metamagnets have been investigated in our previous work.14 And the design of novel cyanoruthenium(III) building blocks continues to be intriguing to us. Phenolic oxime ligands are extensively studied in extractive hydrometallurgy,15 however, the cooperative electronic properties in materials formed by transition metal complexes with cyanide ligands have been overlooked to date.
Herein, a new dicyanoruthenium(III) complex incorporating a relevant phenolic oxime, 2-hydroxyethanoneoxime, is reported. During the reaction, the oxime is reduced to imine by Ru(PPh3)3Cl2 (ref. 16) and thus leading to the formation of complex PPh4[Ru(L)2(CN)2] (L = 2′-hydroxyacetophenone imine). Based on the Ru-containing building block and its relevant hetorometallic units, five cyano-connected heterobimetallic one-dimensional (1D) chain complexes, [Ru(L)2(CN)2Mn(salen)]n (1), [Ru(L)2(CN)2Mn(salcy)]n (2), [Ru(L)2(CN)2Cu(chxn)]n (3), [Ru(L)2(CN)2Mn(nappa)]n (4), and [Ru(L)2(CN)2Mn(napcy)]n (5), have been synthesized and fully studied. In addition, magneto-structural correlation for some typical cyano-bridged heterobimetallic RuIII–MnIII/CuII compounds is accordingly discussed (Scheme 1).
Experimental section
General methods
All reagents and solvents were commercially available and used as received without further purification. [Mn(salen)(H2O)2]ClO4,17 [Mn(salcy)(H2O)2]ClO4,17 [Mn(nappa)(H2O)2]ClO4,17 [Mn(napcy)(H2O)2]ClO4,17 [Cu(chxn)2(H2O)2](NO3)2,18 Me-saoH2 and Ru(L)(PPh3)Cl were prepared according to previously reported methods.16 (Ph4P)[Ru(L)2(CN)2] was carried out by a procedure similar to that for (Bu4N)[Ru(salen)(CN)2] with minor modifications.19![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
Preparation of [Ru(L)2(CN)2Mn(salen)]n (1). A solution of [Mn(salen)(H2O)2]]ClO4 (18.2 mg, 40.0 μmol) in 10 mL of methanol was added to a solution of (Ph4P)[Ru(L)2(CN)2] (35.6 mg, 40.0 μmol) in 10 mL of acetonitrile. After stirring for 10 min, the resulted dark-brown solution was filtered off and then left undisturbed. The slow evaporation of the filtrate at room temperature gave dark-brown rod-like crystals of 1 after two weeks. Yield: 54%. Anal. calcd for C68H56Mn2N12O8Ru2: C, 55.14; H, 3.81; N, 11.35. Found: C, 55.36; H, 3.57; N, 11.01. Selected IR data (KBr, cm−1): 2106 (ν(C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
Preparation of [Ru(L)2(CN)2Mn(salcy)]n (2). Preparation was similar to that of 1 by using [Mn(salcy)(H2O)2]ClO4 (20.4 mg, 40.0 μmol) instead of [Mn(salen)(H2O)2]]ClO4. The slow evaporation of the filtrate resulted in dark-green needles of 2 after two weeks. Yield: 60%. Anal. calcd for C38H32MnN6O4Ru: C, 57.58; H, 4.07; N, 10.60. Found: C, 57.35; H, 4.28; N, 10.85. Selected IR data (KBr, cm−1): 2113 (ν(C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
Preparation of [Ru(L)2(CN)2Cu(chxn)]n (3). Preparation was similar to that of 1 by using Cu(chxn)2(NO3)2 (16.8 mg, 40.0 μmol) instead of [Mn(salen)(H2O)2]]ClO4. The slow evaporation of the filtrate gave dark-green needles of 3 after two weeks. Yield: 60%. Anal. calcd for C48H44CuN12O4Ru2: C, 51.54; H, 3.96; N, 15.03. Found: C, 51.27; H, 4.12; N, 15.22. Selected IR data (KBr, cm−1): 2094 (ν(C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
Preparation of [Ru(L)2(CN)2Mn(nappa)]n (4). Complex 4 was obtained as black block crystals by slow diffusion of a methanol solution (5 mL) of (Ph4P)[Ru(L)2(CN)2] (35.6 mg, 40.0 μmol) and an acetonitrile solution (5 mL) of [Mn(nappa)(H2O)2]ClO4 (22.8 mg, 40.0 μmol) through an H-shaped tube at room temperature for about one month. The resulting crystals were collected, washed with methanol, and dried in air. Yield: 52%. Anal. calcd for C43H34MnN6O4Ru: C, 60.42; H, 4.01; N, 9.83. Found: C, 60.13; H, 4.25; N, 10.01. Selected IR data (KBr, cm−1): 2113 (ν(C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
Preparation of [Ru(L)2(CN)2Mn(napcy)]n (5). Preparation was similar to that of 4, with the exception of [Mn(napcy)(H2O)2]ClO4 (24.4 mg, 40.0 μmol) used instead. The slow evaporation of the filtrate gave dark-green needles of 5 after one month. Yield: 55%. Anal. calcd for C46H32MnN6O4Ru: C, 62.16; H, 3.63; N, 9.46. Found: C, 62.39; H, 3.81; N, 9.21. Selected IR data (KBr, cm−1): 2114 (ν(C
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N)).
N)).
X-ray structure determination
The crystal structures were determined with a Siemens (Bruker) SMART CCD diffractometer using monochromated Mo Kα radiation (λ = 0.71073 Å). The cell parameters were retrieved using SMART software and refined using SAINT20 for all observed reflections. Data were collected using a narrow-frame method with scan widths of 0.30° in ω and an exposure time of 10 s per frame. The absorption corrections were applied using SADABS21 supplied by Bruker. Structures were solved by direct methods using the SHELXL program.22 The positions of metal atoms and their first coordination spheres were located from direct methods E-maps. The other non-hydrogen atoms were found in alternating difference Fourier syntheses and least-squares refinement cycles and, during the final cycles, refined anisotropically. Hydrogen atoms were placed in calculated positions and refined as riding atoms with a uniform value of Uiso. Final crystallographic data and values of R1 and wR2 are listed in Table 1. Selected bond distances and angles for complexes 1–5 are listed in Tables 2–6. CCDC reference numbers are 1400054 (1), 1400055 (2), 1400056 (3), 1400057 (4), and 1400058 (5), respectively (Table 7).| 1 | 2 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| a R1a = Σ||Fo| − |Fc||/Σ|Fo|. wR2b = [Σw(Fo2 − Fc2)2/Σw(Fo2)2]1/2. | |||||
| Formula | C68H60Mn2N12O8Ru2 | C38H34MnN6O4Ru | C48H48CuN12O4Ru2 | C43H36MnN6O4Ru | C46H34MnN6O4Ru |
| fw | 1485.30 | 794.72 | 1122.66 | 856.79 | 890.80 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a, Å | 12.573(2) | 11.3228(8) | 9.9611(13) | 11.2322(11) | 11.680(3) |
| b, Å | 15.090(3) | 13.2197(10) | 12.0243(16) | 12.9655(13) | 13.560(3) |
| c, Å | 21.612(4) | 14.3410(11) | 12.4374(17) | 17.3258(18) | 17.386(4) |
| α, deg | 100.154(3) | 79.9650(10) | 103.509(2) | 84.393(2) | 82.361(4) |
| β, deg | 103.996(3) | 85.0240(10) | 102.368(2) | 81.625(2) | 78.858(4) |
| γ, deg | 94.967(3) | 81.5080(10) | 94.169(2) | 71.103(2) | 67.126(3) |
| V, Å3 | 3880.5 | 2086.5(3) | 1403.0(3) | 2358.3(4) | 2484.2(10) |
| Z | 2 | 2 | 1 | 2 | 2 |
| ρcalcd, g cm−3 | 1.268 | 1.262 | 1.324 | 1.204 | 1.188 |
| T/K | 296(2) | 293(2) | 293(2) | 296(2) | 293(2) |
| μ, mm−1 | 0.754 | 0.706 | 0.955 | 0.630 | 0.600 |
| F(000) | 1504 | 810 | 569 | 874 | 906 |
| Data/restraints/parameters | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 506/0/836 506/0/836 |
7306/0/470 | 6244/24/365 | 8285/0/490 | 8712/0/499 |
| GOF (F2) | 0.932 | 1.179 | 1.061 | 1.143 | 1.103 |
| R1a, wR2b (I > 2σ(I)) | 0.0567, 0.1475 | 0.0591, 0.1392 | 0.0425, 0.1484 | 0.0345, 0.1060 | 0.0655, 0.1723 |
| R1a, wR2b (all data) | 0.0824, 0.1610 | 0.0671, 0.1429 | 0.0459, 0.1541 | 0.0461, 0.1119 | 0.0904, 0.1825 |
| a Symmetry transformations used to generate equivalent atoms: #1: −x + 2, −y + 2, −z + 2; #2: −x + 1, −y + 1, −z. | |||
|---|---|---|---|
| C(9)–Ru(1) | 2.057(5) | C(26)–Ru(2) | 2.067(5) |
| O(5)–Ru(2) | 1.969(3) | Ru(1)–C(9)#1 | 2.057(5) |
| N(1)–Ru(1) | 2.040(4) | N(6)–Ru(2) | 2.037(4) |
| N(7)–Ru(2) | 2.049(4) | Ru(1)–N(1)#1 | 2.040(4) |
| Ru(1)–O(1)#1 | 1.986(3) | Mn(1)–O(2) | 1.902(4) |
| Mn(1)–O(3) | 1.883(4) | Mn(1)–N(4) | 1.989(4) |
| Mn(1)–N(3) | 1.975(4) | Mn(1)–N(5) | 2.298(4) |
| Mn(1)–N(2) | 2.248(4) | Mn(2)–O(6) | 1.879(3) |
| Mn(2)–N(8) | 2.288(4) | N(8)–C(43)–Ru(2) | 178.9(5) |
| N(2)–C(9)–Ru(1) | 177.8(5) | N(5)–C(26)–Ru(2) | 173.9(5) |
| C(9)–N(2)–Mn(1) | 163.6(4) | C(26)–N(5)–Mn(1) | 158.2(5) |
| C(43)–N(8)–Mn(2) | 161.8(4) | C(60)–N(11)–Mn(2) | 159.9(5) |
| C(37)–Ru(1) | 2.062(6) | C(38)–Ru(2) | 2.066(6) |
| Mn(1)–N(1) | 1.994(5) | Mn(1)–N(2) | 2.011(5) |
| Mn(1)–O(1) | 1.864(4) | Mn(1)–O(2) | 1.873(4) |
| Mn(1)–N(5) | 2.396(5) | Mn(1)–N(6) | 2.315(5) |
| N(3)–Ru(2) | 2.045(4) | N(4)–Ru(1) | 2.021(5) |
| O(3)–Ru(2) | 2.005(3) | O(4)–Ru(1) | 1.982(4) |
| N(5)–C(37)–Ru(1) | 174.5(5) | N(6)–C(38)–Ru(2) | 175.4(5) |
| C(37)–N(5)–Mn(1) | 150.1(5) | C(38)–N(6)–Mn(1) | 153.3(4) |
| a Symmetry transformations used to generate equivalent atoms: #1: −x, −y + 2, −z + 1; #2: −x + 1, −y + 2, −z + 1; #3: −x + 1, −y + 1, −z + 1. | |||
|---|---|---|---|
| N(5)–Cu(1) | 2.051(3) | N(6)–Cu(1) | 2.002(4) |
| Cu(1)–N(6′)#2 | 2.002(4) | Cu(1)–N(6)#2 | 2.002(4) |
| Cu(1)–N(5′)#2 | 2.051(3) | Cu(1)–N(5)#2 | 2.051(3) |
| Cu(1)–N(4) | 2.411(4) | Cu(1)–N(4)#2 | 2.411(4) |
| N(1)–Ru(2) | 2.048(4) | N(3)–Ru(1) | 2.046(3) |
| O(1)–Ru(2) | 1.988(3) | O(2)–Ru(1) | 1.982(3) |
| N(4)–C(18)–Ru(1) | 177.9(4) | C(18)–N(4)–Cu(1) | 123.5(3) |
| a Symmetry transformations used to generate equivalent atoms: #1: −x + 2, −y, −z; #2 −x + 1, −y + 1, −z + 1. | |||
|---|---|---|---|
| Ru(1)–O(1)#1 | 1.9904(17) | Ru(1)–O(1) | 1.9904(17) |
| Ru(1)–N(1) | 2.036(2) | Ru(1)–N(1) | 2.036(2) |
| Ru(1)–C(9)#1 | 2.077(3) | Ru(1)–C(9) | 2.077(3) |
| Ru(2)–C(35) | 2.072(3) | Ru(2)–C(35)#2 | 2.072(3) |
| Ru(2)–N(6) | 2.037(2) | Ru(2)–N(6)#2 | 2.037(2) |
| Ru(2)–O(4) | 1.988(2) | Ru(2)–O(4)#2 | 1.988(2) |
| Mn(1)–O(3) | 1.8847(19) | Mn(1)–O(2) | 1.8978(19) |
| Mn(1)–N(3) | 1.971(2) | Mn(1)–N(4) | 1.974(2) |
| Mn(1)–N(5) | 2.292(3) | Mn(1)–N(2) | 2.347(3) |
| N(2)–C(9)–Ru(1) | 178.4(3) | C(35)–N(5)–Mn(1) | 171.9(3) |
| N(5)–C(35)–Ru(2) | 175.3(3) | C(9)–N(2)–Mn(1) | 176.2(3) |
| a Symmetry transformations used to generate equivalent atoms: #1: −x, −y + 1, −z + 2; #2 −x + 1, −y, −z + 1. | |||
|---|---|---|---|
| Mn(1)–O(2) | 1.889(4) | Mn(1)–O(3) | 1.909(4) |
| Mn(1)–N(5) | 1.941(6) | Mn(1)–N(4) | 1.974(5) |
| Mn(1)–N(3) | 2.296(5) | Mn(1)–N(6) | 2.322(5) |
| Ru(1)–O(1)#1 | 1.998(4) | Ru(1)–O(1) | 1.998(4) |
| Ru(1)–N(1) | 2.050(4) | Ru(1)–N(1)#1 | 2.050(4) |
| Ru(1)–C(44) | 2.101(5) | Ru(1)–C(44)#1 | 2.101(5) |
| Ru(2)–O(4)#2 | 1.977(4) | Ru(2)–O(4) | 1.978(4) |
| Ru(2)–C(45)#2 | 2.067(6) | Ru(2)–N(7) | 2.007(6) |
| N(3)–C(44)–Ru(1) | 174.0(5) | N(6)–C(45)–Ru(2) | 177.6(7) |
| C(44)–N(3)–Mn(1) | 172.1(5) | C(45)–N(6)–Mn(1) | 172.9(7) |
| Complexes | dMn–N/Å | Ru–C–N (deg) | Mn–N–C (deg) | Jexp/cm−1 | Ref. |
|---|---|---|---|---|---|
| a H2TPP = meso-tetra(4-phenyl)-porphyrin. | |||||
| Ru(salen)CN2[Mn(L1)] | 2.292–2.307 | 169.9–170.6 | 143.1–144.3 | 1.34 | 13 |
| {[Ru(Q)2](μ-CN)2[Mn(salcy)]}n | 2.286 | 175.7 | 154.2–149.6 | −0.75 | 10a |
| [{Mn(5,5′-Me2salen)}2{Ru(acac)2(CN)2}][Ru(acac)2(CN)2]·2CH3OH | 2.165 | 178.3 | 168.1 | 0.87, 0.24 | 26 |
| {[Ru(acac)2(CN)2][Mn(TPP)]}{[Ph3(PhCH2)P]PF6}2CH3OH | 2.251 | 177.5 | 152.3 | 3.25 | 25 |
| {[Ru(acac)2(CN)2][Mn(TPP)]}{[Ph3(PhCH2)P]ClO4}2CH3OH | 2.250 | 177.7 | 153.0 | 3.43 | 25 |
| [Ru(salen)(CN)2Mn(R,R-salcy)]n | 2.325–2.354 | 174.2–175.4 | 156.4–154.3 | −0.40, −1.32 | 14a |
| [Ru(salen)(CN)2Mn(R,R-salphen)]n | 2.331–2.349 | 168.9–178.0 | 158.7–174.8 | −1.25, −1.87 | 14a |
| 1 | 2.248–2.298 | 177.8 | 158.2–163.6 | −0.37, −0.08 | This paper |
| 2 | 2.315–2.396 | 174.5 | 150.1–153.3 | −3.57, −1.71 | This paper |
| 4 | 2.292–2.347 | 178.4 | 171.9–176.2 | 0.90 | This paper |
| 5 | 2.296–2.322 | 174.0 | 172.9–172.1 | 0.37 | This paper |
Physical measurements
Elemental analyses for C, H, and N were performed on a Perkin-Elmer 240C analyzer. Infrared spectra were recorded on a Vector22 Bruker Spectrophotometer with KBr pellets in the 400–4000 cm−1 region. Magnetic susceptibilities for polycrystalline samples were measured with the use of a Quantum Design MPMS-SQUID-VSM magnetometer in the temperature range of 1.9–300 K. Field dependences of magnetization were measured using Quantum Design MPMS-SQUID-VSM system in an applied field up to 70 kOe.Results and discussion
Syntheses and spectroscopic studies
In this paper, Na[RuIII(L)2(CN)2] was synthesized from the reaction of Ru(L)2(PPh3)Cl and NaCN. After the reaction with Ph4PCl, the dicyanometalate precursor, (PPh4)[RuIII(L)2(CN)2], was prepared in good yield. (PPh4)[RuIII(L)2(CN)2] is soluble in most common organic solvents, such as methanol, acetonitrile, chloroform, and N,N-dimethylformamide. The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching frequency is located at 2096 cm−1, which is comparable to those reported in Ru(III) dicyanides, such as (PPh4)[Ru(Q)2(CN)2] (2095 cm−1),10 Na[RuIII(salen)(CN)2] (2080 cm−1),19 and ((R,R) or (S,S))-[Bu4N][Ru(5-Cl-salcy)](CN)2] (2096 cm−1).14
N stretching frequency is located at 2096 cm−1, which is comparable to those reported in Ru(III) dicyanides, such as (PPh4)[Ru(Q)2(CN)2] (2095 cm−1),10 Na[RuIII(salen)(CN)2] (2080 cm−1),19 and ((R,R) or (S,S))-[Bu4N][Ru(5-Cl-salcy)](CN)2] (2096 cm−1).14
The reactions of (PPh4)[RuIII(L)2(CN)2] and its corresponding metallic counter parts [Mn(SB)(H2O)2]+ or [Cu(chxn)]2+ in methanol (or methanol/acetonitrile) lead to the formation of one-dimensional heterobimetallic complexes 1–5. In IR spectra, C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching vibrations are observed (2106 cm−1 for 1, 2113 cm−1 for 2, 2096 cm−1 for 3, 2113 cm−1 for 4, and 2114 cm−1 for 5), which is consistent with other RuIII–M bimetallic compounds.10a
N stretching vibrations are observed (2106 cm−1 for 1, 2113 cm−1 for 2, 2096 cm−1 for 3, 2113 cm−1 for 4, and 2114 cm−1 for 5), which is consistent with other RuIII–M bimetallic compounds.10a
Structural description
The structural parameters such as key bond lengths and angles are listed in Table 2. The crystal structures of complexes 1–5 are shown in Fig. 1–5, respectively. With the use of different Schiff base ligands, complexes 1, 2, 4, and 5 show similar structures, demonstrated by the crystal cell parameters listed in Table 1 that all crystals sit in the triclinic space group. The asymmetric unit of 1, 2, 4, and 5 is composed of one [MnIII(SB)]+ cation and two [Ru(L)2(CN)2]− anions. For the [MnIII(SB)]+ cations, SB ligands adopt a quasi-planar chelating mode, resulting in the coordination to the MnIII ion along the equatorial plane. Such coordination mode leaves the axial sites available to be trans-coordinated by N atoms from cyanide bridges, thus forming a 1-D (–Ru–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–Mn–N
N–Mn–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–)n liner chain. The axial Mn–Ncyanide bond lengths (2.248(4)–2.396(5) Å) are significantly longer than the equatorial Mn–N(O) (1.883(4)–2.011(5) Å) lengths, forming the elongated octahedral configuration derived from Jahn–Teller distortion of MnIII. The bond angles of Mn–N–Ccyanide are: 158.2(5)–163.6(4)° for 1, 150.1(5)–153.3(4)° for 2, 171.9(3)–176.2(4)° for 4, and 172.1(5)–172.9(7)° for 5, respectively. With regard to the moiety of [Ru(L)2(CN)2]−, the coordination environment of RuIII can also be described as a distorted octahedron, consisting of two N atoms (salen ligand), two C atoms (cyanide groups), and two O atoms (phenolic oxygen). The average Ru–N(O) bond length is 2.015(4) Å, whereas the mean Ru–C length is 2.079(4) Å, which trend agrees well with other RuIII–M bimetallic compounds.10a The Ru–C
C–)n liner chain. The axial Mn–Ncyanide bond lengths (2.248(4)–2.396(5) Å) are significantly longer than the equatorial Mn–N(O) (1.883(4)–2.011(5) Å) lengths, forming the elongated octahedral configuration derived from Jahn–Teller distortion of MnIII. The bond angles of Mn–N–Ccyanide are: 158.2(5)–163.6(4)° for 1, 150.1(5)–153.3(4)° for 2, 171.9(3)–176.2(4)° for 4, and 172.1(5)–172.9(7)° for 5, respectively. With regard to the moiety of [Ru(L)2(CN)2]−, the coordination environment of RuIII can also be described as a distorted octahedron, consisting of two N atoms (salen ligand), two C atoms (cyanide groups), and two O atoms (phenolic oxygen). The average Ru–N(O) bond length is 2.015(4) Å, whereas the mean Ru–C length is 2.079(4) Å, which trend agrees well with other RuIII–M bimetallic compounds.10a The Ru–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N angles (174.0(5)–178.4(3)°) show slight deviation from linearity. With respect to the molecular chains, complex 1, for instance, is running along the b axis with the intrachain Ru⋯Mn separation of 5.457(3) Å through the bridging cyanide. The shortest interchain Ru⋯Ru, Mn⋯Mn, and Ru⋯Mn distances are 7.232, 6.732, and 8.099 Å, respectively, obviously longer than the intrachain metal⋯metal distances.
N angles (174.0(5)–178.4(3)°) show slight deviation from linearity. With respect to the molecular chains, complex 1, for instance, is running along the b axis with the intrachain Ru⋯Mn separation of 5.457(3) Å through the bridging cyanide. The shortest interchain Ru⋯Ru, Mn⋯Mn, and Ru⋯Mn distances are 7.232, 6.732, and 8.099 Å, respectively, obviously longer than the intrachain metal⋯metal distances.
 | ||
| Fig. 1 Asymmetric unit (top) and one-dimensional chain structure (bottom) of complex 1. Hydrogen atoms are omitted for clarity. | ||
 | ||
| Fig. 2 Asymmetric unit (top) and one-dimensional chain structure (bottom) of complex 2. Hydrogen atoms are omitted for clarity. | ||
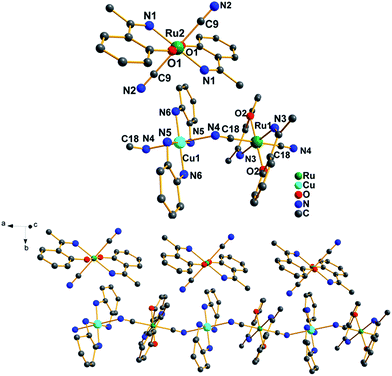 | ||
| Fig. 3 Asymmetric cationic unit (top) and one-dimensional cationic chain structure (bottom) of complex 3. Hydrogen atoms are omitted for clarity. | ||
 | ||
| Fig. 4 Asymmetric unit (top) and one-dimensional chain structure (bottom) of complex 4. Hydrogen atoms are omitted for clarity. | ||
 | ||
| Fig. 5 Asymmetric unit (top) and one-dimensional chain structure (bottom) of complex 5. Hydrogen atoms are omitted for clarity. | ||
There is no significant interaction between two neighboring chains except for 4. Each chain interacts with the two adjacent chains via π–π stacking between aromatic rings of the salcy ligands with a centroid distance of 3.353 Å, thus forming the 3D structure (Fig. S1†).
Treatment of Cu(chxn)2(NO3)2 with trans-(Ph4P)[Ru(L)2(CN)2] in methanol at room temperature gives complex 3 (Fig. 3). Complex 3 displays a single cationic chain structure consisting of a [Cu(chxn)2][Ru(L)2(CN)2]n unit and a free [Ru(L)2(CN)2]− anion. The CuII center has a distorted octahedral environment coordinated by four equatorial nitrogen atoms from two chxn ligands and two axial nitrogen atoms from [Ru(L)2(CN)2]− units with a trans configuration. The Cu–Ncyanide length of 2.411(4) Å is slightly longer than the Cu–Nchxn lengths [2.002(4) and 2.051(3) Å]. The bridging Ru–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N angle is 177.9(4)°. The bond lengths in each [Ru(L)2(CN)2]− unit [Ru–O of 1.982(3)–1.988(3) Å and Ru–N of 2.046(4)–2.048(3) Å] are similar to those found in trans-(Ph4P)[Ru(acac)2(CN)2].10a Furthermore, the intramolecular Cu⋯Ru distance is 4.981 Å, along with the Cu–N–C–Ru torsion angle of −160.2° and the closest intermolecular Ru⋯Ru separation of 6.012 Å.
N angle is 177.9(4)°. The bond lengths in each [Ru(L)2(CN)2]− unit [Ru–O of 1.982(3)–1.988(3) Å and Ru–N of 2.046(4)–2.048(3) Å] are similar to those found in trans-(Ph4P)[Ru(acac)2(CN)2].10a Furthermore, the intramolecular Cu⋯Ru distance is 4.981 Å, along with the Cu–N–C–Ru torsion angle of −160.2° and the closest intermolecular Ru⋯Ru separation of 6.012 Å.
Magnetic properties
Magnetic susceptibility measurements were performed on polycrystalline samples of complexes 1–5 using the SQUID magnetometer at temperatures ranging from 1.9 K to 300 K.According to the structures, the RuIII–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–MnIII linkages are obviously different. An alternating ferrimagnetic chain model
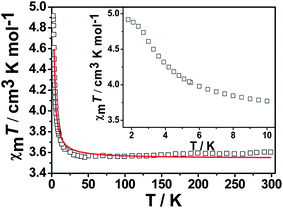
N–MnIII linkages are obviously different. An alternating ferrimagnetic chain model  was used,23 where the local spins are SRu and SMn, the local Zeeman factors gRu and gMn, and the couplings between nearest neighbors J(1 + α) and J(1 − α). The best fit between 30 and 300 K gives gMn = 2.01(1), gRu = 1.96(1), J = −0.23(1) cm−1, α = 0.63(1), and zJ′ = 0.12(3) (R = 3.64 × 10−4) for 1 and gMn = 1.95(1), gRu = 1.99(1), J = −2.67(1) cm−1, α = −0.34(1), and zJ′ = −0.56(2) (R = 1.16 × 10−4) for 2, where R = ∑[(χMT)obs − (χMT)calc]2/∑(χMT)obs2. The above mentioned result confirms that antiferromagnetic couplings between RuIII and MnIII ions within the chain are observed.
was used,23 where the local spins are SRu and SMn, the local Zeeman factors gRu and gMn, and the couplings between nearest neighbors J(1 + α) and J(1 − α). The best fit between 30 and 300 K gives gMn = 2.01(1), gRu = 1.96(1), J = −0.23(1) cm−1, α = 0.63(1), and zJ′ = 0.12(3) (R = 3.64 × 10−4) for 1 and gMn = 1.95(1), gRu = 1.99(1), J = −2.67(1) cm−1, α = −0.34(1), and zJ′ = −0.56(2) (R = 1.16 × 10−4) for 2, where R = ∑[(χMT)obs − (χMT)calc]2/∑(χMT)obs2. The above mentioned result confirms that antiferromagnetic couplings between RuIII and MnIII ions within the chain are observed.
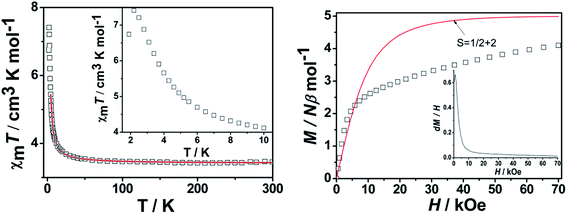
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bridges in 3.10a In order to gain further insights into the underlying magnetic nature of 3, the field dependence of magnetization was measured at 1.90 K (Fig. 9). The magnetization increases slowly to a maximum value of 2.82 Nβ at 70 kOe, which is smaller than the expected value of 3.00 Nβ with the antiferromagnetic coupling between RuIII and MnIII ions. Alternating current magnetic measurements (Fig. S5†) show undetectable frequency-dependent χM′ and χM′′ signals for 3, indicating a completely paramagnetic behavior without any magnetic ordering.
N bridges in 3.10a In order to gain further insights into the underlying magnetic nature of 3, the field dependence of magnetization was measured at 1.90 K (Fig. 9). The magnetization increases slowly to a maximum value of 2.82 Nβ at 70 kOe, which is smaller than the expected value of 3.00 Nβ with the antiferromagnetic coupling between RuIII and MnIII ions. Alternating current magnetic measurements (Fig. S5†) show undetectable frequency-dependent χM′ and χM′′ signals for 3, indicating a completely paramagnetic behavior without any magnetic ordering.
Between 4.5 K and 300 K, the best least-squares fit yields g = 2.01(1), J = 0.90(1) cm−1, with R = 2.5 × 10−4 for complex 4. The susceptibility data were fitted over 4.5 K using the same expression that gives g = 2.05(1), J = 0.37(1) cm−1, with R = 8.7 × 10−4 for complex 5. The fitting results imply that the effect of ferromagnetic couplings between RuIII and MnIII ions in 4 is stronger than those in 5.
Field-dependent magnetizations for 4 and 5 were measured from 0–70 kOe at 1.9 K (Fig. 9 and S9†). As the intensity of applied field increases, the magnetizations continuously increase until reaching 4.10 Nβ and 3.95 Nβ at 70 kOe for 4 and 5, respectively, which are slightly lower than the ferromagnetic result (5.00 Nβ) calculated from Ms = g(SRu + SMn) with g = 2.00.
To gain further insight into the underlying magnetic nature of 4, from the dM/dH vs. H plot at 1.90 K (inset of Fig. 9), the critical field is estimated to be about 920 kOe. To investigate the phase transition at low temperature, ZFC and FC curves show an abrupt increase below 2.20 K with a divergence (Fig. 11), suggesting the occurrence of a long-range magnetic ordering below this temperature. Furthermore, alternating current (ac) magnetic susceptibilities measurements were carried out at zero direct current (dc) field (Fig. 11). The results show that only in-phase (χM) signals display a peak around 2.20 K, demonstrating the field-induced three-dimensional antiferromagnetic ordering below this temperature.
It is noted that the related magneto-structurally characterized analogues based on RuIII–M systems were found to be either ferro- or antiferromagnetic coupling systems.10,13,14,25,26 According to Kahn's orbital symmetry model,20 the nature of Cu(II) and Ru(III) interaction should be ferromagnetic (JF). The observed antiferromagnetic interaction (JAF) of CuII and RuIII via cyano bridges in 3 may be ascribed to the very acute Cu–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C angle (123.5(3)°) and long Cu⋯Ru distance (4.981 Å). A similar antiferromagnetic interaction (JAF) has also been observed in the compound {[RuIII(Q)2(CN)]2(μ-CN)2[CuII(cyclam)]}.10a In previous studies, the ferromagnetic coupling could be universally observed in cyanide-bridged Ru(III)–Mn(III) systems.25 For the nature of RuIII and MnIII interaction, it is expected that when the dπ orbitals on RuIII and MnIII are overlapped, antiferromagnetic interactions (JAF) will emerge and vice versa. As shown in Table 6, the most complexes are in accordance with the above theoretical analysis. The magnetic behaviors of complexes 1, 2, 4, and 5 appear to be slightly unusual. Especially, the bond lengths of Mn–N(cyanide) in 4 (2.292(3)–2.347(3) Å) and 5 (2.296(5)–2.322(5) Å) are in accord with those in [RuIII(salen)(CN)2][MnIII(L2)]13 (L2 = N,N′-(1-methylethylene)bis(2-hydroxynaphthalene-1-carbaldehydeneiminate)dianion); and the bond angles of Mn–N
C angle (123.5(3)°) and long Cu⋯Ru distance (4.981 Å). A similar antiferromagnetic interaction (JAF) has also been observed in the compound {[RuIII(Q)2(CN)]2(μ-CN)2[CuII(cyclam)]}.10a In previous studies, the ferromagnetic coupling could be universally observed in cyanide-bridged Ru(III)–Mn(III) systems.25 For the nature of RuIII and MnIII interaction, it is expected that when the dπ orbitals on RuIII and MnIII are overlapped, antiferromagnetic interactions (JAF) will emerge and vice versa. As shown in Table 6, the most complexes are in accordance with the above theoretical analysis. The magnetic behaviors of complexes 1, 2, 4, and 5 appear to be slightly unusual. Especially, the bond lengths of Mn–N(cyanide) in 4 (2.292(3)–2.347(3) Å) and 5 (2.296(5)–2.322(5) Å) are in accord with those in [RuIII(salen)(CN)2][MnIII(L2)]13 (L2 = N,N′-(1-methylethylene)bis(2-hydroxynaphthalene-1-carbaldehydeneiminate)dianion); and the bond angles of Mn–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(cyanide) (171.9(3)–176.2(4)°) for 4 and 172.1(5)–172.9(7)° for 5 are remarkably larger than those in [RuIII(salen)(CN)2][MnIII(L2)].13 Magnetic investigation reveals ferromagnetic coupling in 4 and 5. The t2g orbitals of RuIII and MnIII overlap efficiently, resulting in the un-production of antiferromagnetic coupling and its mechanism for a magnetic exchange in the pathway of RuIII–C
C(cyanide) (171.9(3)–176.2(4)°) for 4 and 172.1(5)–172.9(7)° for 5 are remarkably larger than those in [RuIII(salen)(CN)2][MnIII(L2)].13 Magnetic investigation reveals ferromagnetic coupling in 4 and 5. The t2g orbitals of RuIII and MnIII overlap efficiently, resulting in the un-production of antiferromagnetic coupling and its mechanism for a magnetic exchange in the pathway of RuIII–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N–MnIII was well analyzed by Lau et al. through DFT calculations.26 In conclusion, both the relative symmetries and relative energies of the magnetic orbitals are valuable in determining the overall magnetic coupling in bimetallic assemblies. Therefore, the full understanding of this magnetic behavior induced by energies of the magnetic orbitals deserves further investigation.
N–MnIII was well analyzed by Lau et al. through DFT calculations.26 In conclusion, both the relative symmetries and relative energies of the magnetic orbitals are valuable in determining the overall magnetic coupling in bimetallic assemblies. Therefore, the full understanding of this magnetic behavior induced by energies of the magnetic orbitals deserves further investigation.
Conclusions
Five 1-D zigzag RuIII–MnIII/CuII chains obtained on a basis of a new paramagnetic ruthenium(III)-derived building block, trans-[Ru(L)2(CN)2]−, have been synthesized and structurally characterized. Complexes 1–3 exhibit intrachain antiferromagnetic coupling between RuIII and MnIII/CuII entities through cyanide ligands, while intrachain ferromagnetic coupling between RuIII and MnIII through cyanide ligands can be observed for complexes 4 and 5. It is noteworthy that complex 4 shows interesting metamagnetic behaviors with a critical field of about 920 Oe at 1.9 K. Furthermore, magneto-structural correlation for some typical cyano-bridged heterobimetallic RuIII–MnIII/CuII compounds has been discussed in this work. It can be assumed that the relative symmetries and their corresponding magnetic orbital energies are valuable in determining the overall magnetic coupling in bimetallic assemblies. On the basis of the above mentioned results, the present work shows that the type of [Ru(L)2(CN)2]− building block is useful for the construction of low-dimensional 3d–4d magnetic materials.Abbreviations
| salen | N,N′-Ethylenebis(salicylideneimine)dianion |
| salcy | N,N′-(1,2-Cyclohexanediylethylene)bis(salicylideneiminato)dianion |
| chxn | 1,2-Diaminocyclohexane |
| nappa | N,N′-(1-Methylethylene)bis(2-hydroxynaphthalene-1-carbaldehydene-iminate)dianion |
| napcy | N,N′-(1,2-Cyclohexanediylethylene)bis(2-hydroxynaphthalene-1-carbaldehydene-iminate)dianion |
Acknowledgements
This work was supported by the Major State Basic Research Development Program (2013CB922101), the National Natural Science Foundation of China (51173075), and the Natural Science Foundation of Jiangsu Province (BK20130054). We also thank Dr Tian-Wei Wang for the experimental assistance on magnetic measurements.References
- (a) S. Wang, J. L. Zuo, H. C. Zhou, H. J. Choi, Y. X. Ke, J. R. Long and X. Z. You, Angew. Chem., Int. Ed., 2004, 43, 5940–5943 CrossRef CAS PubMed; (b) K. Qian, X. C. Huang, C. Zhou, X. Z. You, X. Y. Wang and K. R. Dunbar, J. Am. Chem. Soc., 2013, 135, 13302–13305 CrossRef CAS PubMed; (c) K. S. Pedersen, J. Bendix and R. Clerac, Chem. Commun., 2014, 50, 4396–4415 RSC; (d) N. Hoshino, F. Iijima, G. N. Newton, N. Yoshida, T. Shiga, H. Nojiri, A. Nakao, R. Kumai, Y. Murakami and H. Oshio, Nat. Chem., 2012, 4, 921–926 CrossRef CAS PubMed; (e) D. P. Dong, T. Liu, S. Kanegawa, S. Kang, O. Sato, C. He and C. Y. Duan, Angew. Chem., Int. Ed., 2012, 51, 5119–5123 CrossRef CAS PubMed; (f) K. J. Cho, D. W. Ryu, H. Y. Kwak, J. W. Lee, W. R. Lee, K. S. Lim, E. K. Koh, Y. W. Kwon and C. S. Hong, Chem. Commun., 2012, 48, 7404–7406 RSC.
- (a) X. Y. Wang, C. Avendano and K. R. Dunbar, Chem. Soc. Rev., 2011, 40, 3213–3238 RSC; (b) R. Lescouëzec, L. M. Toma, J. Vaissermann, M. Verdaguer, F. S. Delgado, C. Ruiz-Pérez, F. Lloret and M. Julve, Coord. Chem. Rev., 2005, 249, 2691–2729 CrossRef; (c) L. M. Baraldo, P. Forlano, A. R. Parise, L. D. Slep and J. A. Olabe, Coord. Chem. Rev., 2001, 219, 881–921 CrossRef.
- S. Wang, X. H. Ding, Y. H. Li and W. Huang, Coord. Chem. Rev., 2012, 256, 439–464 CrossRef CAS.
- X. Feng, J. Liu, T. D. Harris, S. Hill and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7521–7529 CrossRef CAS PubMed.
- J. M. Zadrozny, D. E. Freedman, D. M. Jenkins, T. D. Harris, A. T. Iavarone, C. Mathoniere, R. Clerac and J. R. Long, Inorg. Chem., 2010, 49, 8886–8896 CrossRef CAS PubMed.
- (a) T. S. Venkatakrishnan, S. Sahoo, N. Brefuel, C. Duhayon, C. Paulsen, A. L. Barra, S. Ramasesha and J. P. Sutter, J. Am. Chem. Soc., 2010, 132, 6047–6056 CrossRef CAS PubMed; (b) D. Pinkowicz, R. Podgajny, R. Pelka, W. Nitek, M. Balanda, M. Makarewicz, M. Czapla, J. Zukrowski, C. Kapusta, D. Zajac and B. Sieklucka, Dalton Trans., 2009, 7771–7777 RSC; (c) B. Yan, H. Zhou and A. Lachgar, Inorg. Chem., 2003, 42, 8818–8822 CrossRef CAS PubMed.
- (a) M. Perrier, J. Long, F. A. Paz, Y. Guari and J. Larionova, Inorg. Chem., 2012, 51, 6425–6427 CrossRef CAS PubMed; (b) S. Ren, S.-L. Ma, G.-F. Xu, B. Gu, Y. Ma, Q.-L. Wang and D.-Z. Liao, Inorg. Chem. Commun., 2011, 14, 1124–1127 CrossRef CAS; (c) R. Podgajny, S. Choraży, W. Nitek, A. Budziak, M. Rams, C. J. Gómez-García, M. Oszajca, W. Łasocha and B. Sieklucka, Cryst. Growth Des., 2011, 11, 3866–3876 CrossRef CAS; (d) J. Long, E. Chelebaeva, J. Larionova, Y. Guari, R. A. Ferreira, L. D. Carlos, F. A. Almeida Paz, A. Trifonov and C. Guerin, Inorg. Chem., 2011, 50, 9924–9926 CrossRef CAS PubMed.
- (a) D. Visinescu, R. Jeon Ie, A. M. Madalan, M. G. Alexandru, B. Jurca, C. Mathoniere, R. Clerac and M. Andruh, Dalton Trans., 2012, 41, 13578–13581 RSC; (b) M. G. Alexandru, D. Visinescu, A. M. Madalan, F. Lloret, M. Julve and M. Andruh, Inorg. Chem., 2012, 51, 4906–4908 CrossRef CAS PubMed; (c) D. Li, L. Zheng, Y. Zhang, J. Huang, S. Gao and W. Tang, Inorg. Chem., 2003, 42, 6123–6129 CrossRef CAS PubMed.
- (a) E. V. Peresypkina, D. G. Samsonenko and K. E. Vostrikova, J. Solid State Chem., 2015, 224, 107–114 CrossRef CAS; (b) D. G. Samsonenko, C. Paulsen, E. Lhotel, V. S. Mironov and K. E. Vostrikova, Inorg. Chem., 2014, 53, 10217–10231 CrossRef CAS PubMed; (c) M. R. Saber and K. R. Dunbar, Chem. Commun., 2014, 50, 2177–2179 RSC.
- (a) J. Xiang, L. H. Jia, B. W. Wang, S. M. Yiu, S. M. Peng, W. Y. Wong, S. Gao and T. C. Lau, Dalton Trans., 2013, 42, 3876–3887 RSC; (b) W. F. Yeung, W. L. Man, W. T. Wong, T. C. Lau and S. Gao, Angew. Chem., Int. Ed., 2001, 40, 3031–3033 CrossRef CAS.
- W. F. Yeung, P. H. Lau, T. C. Lau, H. Y. Wei, H. L. Sun, S. Gao, Z. D. Chen and W. T. Wong, Inorg. Chem., 2005, 44, 6579–6590 CrossRef CAS PubMed.
- G. Marinescu, C. Maxim, R. Clérac and M. Andruh, Inorg. Chem., 2015, 51, 5621–5623 CrossRef PubMed.
- J. H. Yoon, H. S. Yoo, H. C. Kim, S. W. Yoon, B. J. Suh and C. S. Hong, Inorg. Chem., 2009, 48, 816–818 CrossRef CAS PubMed.
- (a) J. Ru, F. Gao, M. X. Yao, T. Wu and J. L. Zuo, Dalton Trans., 2014, 43, 18047–18055 RSC; (b) J. Ru, F. Gao, T. Wu, M. X. Yao, Y. Z. Li and J. L. Zuo, Dalton Trans., 2014, 43, 933–936 RSC; (c) L. Cui, F. F. Zhu, C. F. Leong, J. Ru, F. Gao, D. M. D'Alessandro and J. L. Zuo, Sci. China: Chem., 2015, 58, 650–657 CrossRef CAS.
- (a) A. G. Smith, P. A. Tasker and D. White, Coord. Chem. Rev., 2003, 241, 61–85 CrossRef CAS; (b) P. Tasker and V. Gasperov, Macrocyclic Chem., 2005, 365–382 CAS; (c) J. Szymanowski, Hydroxyoximes and Copper Hydrometallurgy, CRC Press, Boca Raton, FL, 1993 Search PubMed.
- A. Kumar Das, S. M. Peng and S. Bhattacharya, J. Chem. Soc., Dalton Trans., 2000, 181–184 Search PubMed.
- (a) M. X. Yao, Q. Zheng, X. M. Cai, Y. Z. Li, Y. Song and J. L. Zuo, Inorg. Chem., 2012, 51, 2140–2149 CrossRef CAS PubMed; (b) M. X. Yao, Q. Zheng, F. Gao, Y. Z. Li and J. L. Zuo, Sci. China: Chem., 2012, 55, 1022–1030 CrossRef CAS.
- C. F. Wang, D. P. Li, X. Chen, X. M. Li, Y. Z. Li, J. L. Zuo and X. Z. You, Chem. Commun., 2009, 6940–6942 RSC.
- W. H. Leung and C. M. Che, Inorg. Chem., 1989, 28, 4619–4622 CrossRef CAS.
- SAINT-Plus, version 6.02, Bruker Analytical X-ray System, Madison, WI, 1999 Search PubMed.
- G. M. Sheldrick, SADABS an empirical absorption correctionprogram, Bruker Analytical X-ray Systems, Madison, WI, 1996 Search PubMed.
- G. M. Sheldrick, SHELXTL-97, Universität of Göttingen, Göttingen, Germany, 1997 Search PubMed.
- O. Kahn, Molecular Magnetism, VCH, Weinhem, Germany, 1993 Search PubMed.
- J. Seiden, J. Phys., Lett., 1983, 44, L947–L952 CrossRef.
- D. P. Zhang, L. F. Zhang, Y. T. Chen, H. L. Wang, Z. H. Ni, W. Wernsdorferc and J. Z. Jiang, Chem. Commun., 2010, 46, 3550–3552 RSC.
- J.-F. Guo, X.-T. Wang, B.-W. Wang, G.-C. Xu, S. Gao, L. Szeto, W.-T. Wong, W.-Y. Wong and T.-C. Lau, Chem.–Eur. J., 2010, 16, 3524–3525 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional structures, spectroscopic data, magnetic characterization data, and X-ray crystallographic files in CIF formats for 1–5. CCDC 1400054–1400058. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra15752h |
| This journal is © The Royal Society of Chemistry 2015 |