A {110} facet predominated Bi6O6(OH)3(NO3)3·1.5H2O photocatalyst: selective hydrothermal synthesis and its superior photocatalytic activity for degradation of phenol†
Abstract
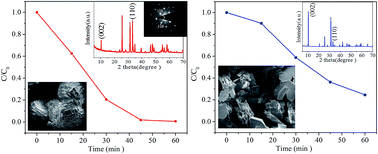
A basic bismuth(III) nitrate photocatalyst with the composition of Bi6O6(OH)3(NO3)3·1.5H2O (BBN) was facilely synthesized using a hydrothermal strategy via incomplete hydrolysis of bismuth nitrate. Characterization of the composition, morphology, microstructure, optical absorption, BET surface area, and photocatalytic behavior was systematically explored. The results indicated that BBN architectures built up of multilayered meshing-teeth structures with predominant {110} side facets can be selectively obtained by fine-tuning the reaction parameters. The sample exhibits an obviously superior photocatalytic activity for the degradation of phenol compared with BBN sheets with dominant top {001} planes and commercial P25, with the rate constant k improved by 3.6 and 2.8 fold, respectively. The excellent photocatalytic behavior combined with the rather low BET surface area of 0.0453 m2 g−1 indicate that the highly reactive {110} facets in BBN are responsible for the photocatalysis. The active oxidation species and main intermediates in the phenol/BBN system are ascertained using scavenger experiments and high performance liquid chromatography (HPLC) techniques. Combining the band edge of BBN and the redox potentials of the active species, a possible migration mechanism of photogenerated e−/h+ pairs on the surface of BBN is proposed. This work provides some new insights for the rational design and synthesis of active-facet exposed basic salt photocatalysts with excellent efficiency.

 Please wait while we load your content...
Please wait while we load your content...