Photoluminescence properties, crystal structure and electronic structure of a Sr2CaWO6:Sm3+ red phosphor
Abstract
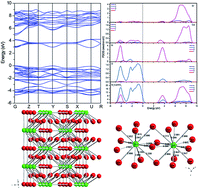
A novel Sm3+-doped Sr2CaWO6 (SCWO) red phosphor, synthesized though a solid-state reaction, was reported. Its crystal structure was analyzed and refined via the Rietveld full-pattern fitting method based on XRD patterns. The CASTEP module of Materials Studio was used to investigate the band structure and density of states of the SCWO. The optical band gap was calculated through the UV-vis diffuse reflectance spectrum and compared with the value predicted by the DFT method. Raman spectra were recorded to confirm the substitution of cations by Sm3+ ions. The broad W–O charge transfer band and narrow excitation band at 406 nm from Sm3+ ions have comparable intensities in SCWO:Sm3+. After the introduction of charge compensators Li+, Na+ or K+, the intensity of the near-UV excitation band at 406 nm was almost twice as much as before. The profiles of the emission spectra under excitation into the charge transfer absorption and f–f transition of Sm3+ are different, and low-symmetry Sm3+ centres are preferentially excited via f–f absorption transitions. The intensity ratios of electronic, magnetic dipole-allowed transitions under different radiations show that the charge compensators can influence the chemical environment around Sm3+.

 Please wait while we load your content...
Please wait while we load your content...