Porous PLGA microparticles to encapsulate doxorubicin and polyethylenimine/miR-34a for inhibiting the proliferation and migration of lung cancer†
Chenhui Wang‡
,
Di Wu‡,
Jiebing Yang,
Haobo Han,
Zhen Xing,
Yan Zhang,
Yan Yang* and
Quanshun Li*
Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, School of Life Sciences, Jilin University, Changchun 130012, China. E-mail: quanshun@jlu.edu.cn; yyan@jlu.edu.cn; Fax: +86-431-85155200; Tel: +86-431-85155216
First published on 21st September 2015
Abstract
Porous PLGA microparticles were successfully prepared for achieving the co-delivery of doxorubicin and PEI25K/miR-34a, using ammonium bicarbonate as a porogen. The synergistic effect between these two components ensured the efficient anti-proliferative and anti-migration effects of porous PLGA microparticles on tumor cells.
In recent years, lung cancer has been a leading cause of malignancy-related death in both developing and developed countries.1,2 Conventional modalities, such as chemotherapy and radiotherapy, are the major treatment strategies for patients bearing lung cancer.3 However, severe toxicities and undesirable side effects can't be avoided as the anticancer agents act on both normal and tumor cells. Compared with these methods, gene therapy could directly alleviate the abnormalities arising from mutation or changes in gene expression, and thus it has been considered as a potential treatment strategy for tumors.4
In tumor gene therapy, microRNA (miRNA)-based therapy is a promising technique to solve the tremendous challenges in cancer treatment.5 miRNAs are a type of small endogenous noncoding RNAs which regulate the stability and translation of mRNAs, and tumor suppressor miRNAs are usually of low expression in tumor cells.6 Improving the expression level of tumor suppressive miRNAs could be employed to achieve the anti-tumor function by inducing cell apoptosis or inhibiting the migration and invasion of tumor cells.7,8 Among them, miR-34a is an endogenous miRNA regulated by p53 network at transcriptional level, which plays a key role in inhibiting the genesis and progression of human cancers.9–11 Since miR-34a is commonly down-regulated in many human tumors, enhanced expression of miR-34a has been demonstrated to be a potential strategy for tumor gene therapy,12 and it can even overcome the tumors' poor responses to cytotoxic drugs and exert synergistic effect with chemotherapeutics.13 Thus, the co-delivery of miR-34a and small molecular agents through polymeric carriers will be favorable for improving the overall outcomes in cancer treatment.
Another problem to be solved in the lung cancer treatment is the low delivery efficiency of intravenously-administered agents to the lungs.14 Compared with the intravenous administration, inhalatory route does not need to produce high systemic level of therapeutic agents, which is beneficial for reducing the adverse side effects.15–17 In the inhalatory delivery systems, polymer-based porous microparticles have been demonstrated to be suitable for pulmonary drug administration, owing to their low densities and aerodynamic diameters, and favorable lung deposition profiles.18–22 To date, porous microparticles have been extensively explored for delivering payloads to the lungs, including small molecules, proteins and plasmid DNA.23–27 In our previous report, using PLGA and PEI25K as carriers, porous microparticles have been successfully constructed to realize the co-delivery of doxorubicin and p53 gene, which exhibited good anti-proliferative and apoptosis-inducing activity in lung cancer cells.28 Compared with p53 gene, miR-34a regulated by p53 network could further solve the drug resistance of tumor cells via inhibiting the expression level of Bcl-2 and achieve the anti-migration and anti-invasion effects via targeting Notch-1 and MMP-9.13 Thus, multiple targeting ability of tumor suppressive miR-34a made it more promising to improve the tumor treatment, especially for creating synergistic effects with other therapeutic approaches.
Herein, the water–oil–water emulsion solvent evaporation method was employed to prepare porous PLGA microparticles using ammonium bicarbonate as a porogen, namely blank porous PLGA microparticles (MP-1), porous PLGA microparticles loaded with PEI25K/miR-34a (MP-2), porous PLGA microparticles loaded with doxorubicin (MP-3) and porous PLGA microparticles loaded with doxorubicin and PEI25K/miR-34a (MP-4). As shown in Fig. 1, the PLGA microparticles exhibited considerable surface porosity, which was caused by the decomposition of ammonium bicarbonate into ammonia and carbon dioxide, and also good dispersion with a mean particle size of ca. 40 μm. The particle size and zeta potential of porous PLGA microparticles were then determined (Table S1†). The aerodynamic diameters of these porous microparticles were determined to be <10 μm, and thus they could be capable of efficient alveolar deposition for a long time if they were well-aerosolized and properly inhaled. The loading of doxorubicin and PEI25K/miR-34a had almost no influence on the particle size, with a size of 43.4–48.9 μm. Meanwhile, the MP-1, MP-3 and MP-4 microparticles were of negative zeta potentials, whereas MP-2 showed a positively charged state due to the encapsulation of positive PEI25K/miR-34a nanocomplex. The drug loading values of doxorubicin in MP-3 and MP-4 microparticles were 0.823% and 0.773%, respectively, reflecting high encapsulation efficiencies (77–82%). Nevertheless, the loading of doxorubicin would reduce the encapsulation efficiency of miR-34a (33.5%), attributing to the competition effect between these two components during the preparation of porous microparticles.
 | ||
| Fig. 1 SEM analysis of the porous PLGA microparticles MP-4 (A) loaded with doxorubicin and PEI25K/miR-34a and their porous surface (B). | ||
To get a direct evidence for the co-encapsulation of doxorubicin and miR-34a, porous PLGA microparticles were observed by confocal laser scanning microscopy (Fig. S1†). The microparticles showed a porous structure, and red fluorescence (doxorubicin) and green fluorescence (FITC-labeled PEI25K/miR-34a) could be clearly observed, suggesting the successful co-encapsulation of doxorubicin and miR-34a. In the release profiles, porous microparticles showed a sustained release manner of doxorubicin and miR-34a due to the decomposition of PLGA in PBS, and the co-encapsulation of these two components did not affect their individual release behavior (Fig. S2†). After 30 days, the cumulative release values of doxorubicin and miR-34a were measured to be 80% and 60%, respectively. Meanwhile, PEI25K/miR-34a nanoparticles were remained in the release supernatants (Fig. S3†), which was beneficial for achieving efficient endocytosis and further transfection.
The anti-tumor efficiency of porous PLGA microparticles was evaluated by MTT assay, using human lung adenocarcinoma cell line A549 as a model. As shown in Fig. 2, the release supernatant from MP-4 at 30 days exhibited a stronger anti-proliferative effect than the corresponding samples from MP-2 and MP-3, which meant the miR-34a loading in porous microparticles could potentially reduce the dosage of chemotherapeutics during cancer therapy. Notably, the cell viability could be reduced to ca. 40% after treating with the release supernatant from MP-4 at 30 days for 24 h. Similar phenomenon could be observed in the groups with 48 h treatment (Fig. S4†). The morphological analysis using DAPI staining indicated the presence of chromatin condensation and the formation of apoptotic bodies after treating with the release supernatant of MP-4 (Fig. S5†).
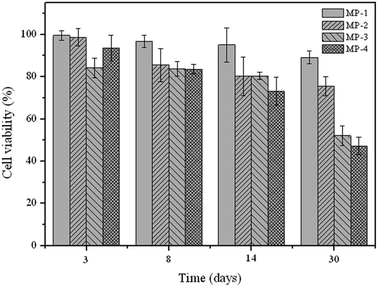 | ||
| Fig. 2 Cell viability of A549 cells after treating with the release supernatants at 30 days from porous PLGA microparticles for 24 h. Data were represented as mean value ± SD, n = 4. | ||
The anti-proliferative mechanism, including apoptosis induction and cell cycle arrest, was systematically analyzed by flow cytometry. As shown in Fig. 3, the largest population of apoptotic cells has been detected in the release supernatant of MP-4 group (59.97%), which was mainly caused by the release of doxorubicin. Though miR-34a released from porous PLGA microparticles could slightly trigger the cell apoptosis (7.12%), it could facilitate the doxorubicin-inducing cell apoptosis, implying an obvious synergistic effect between doxorubicin and miR-34a. Thus, the porous PLGA microparticles loaded with both doxorubicin and miR-34a substantially outperformed the ones harboring a single agent in the induction of apoptosis. To further elucidate the apoptosis-inducing mechanism, the expression level of proteins associated with cell apoptosis was detected through western blotting analysis (Fig. 4). The activation of procaspase-3 to caspase-3 to generate the active executive caspase was clearly observed in A549 cells treated with the release supernatant of MP-4, much stronger than MP-2 and MP-3 groups. In addition, the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) was efficiently cleaved owing to the activation of procaspase-3. The Bcl-2 expression exhibited a slightly decreased tendency compared to the control, suggesting that the cells treated with the release supernatant would produce a decreased anti-apoptotic barrier, and thus it would be favorable for overcoming the multi-drug resistance of tumor cells. Interestingly, the expression level of caspase-9 showed an obviously improved state, whereas caspase-8 expression was independent on the treatment of release supernatant. The results were consistent with the activity measurement of caspase-3, 8 and 9 (Fig. S6†), which also indicated the activation of caspase-3 and caspase-9 and no changes in caspase-8. For cell cycle arrest analysis, porous PLGA microparticles MP-3 and MP-4 could arrest the cell growth by holding the cell cycle at G2 phase in a similar level (37.76% and 37.26%) (Fig. 5 and S7†). The miR-34a loading had almost no effects on the cell cycle phase, and thus the cell cycle arrest was mainly attributed to the function of doxorubicin. Notably, compared with MP-2 and MP-3 groups, an increased ratio of sub-G1 phase could be clearly detected in the MP-4 group, indicating the stronger induction of cell apoptosis. The results were in coincidence with cell apoptosis analysis, also suggesting that miR-34a did not trigger the cell cycle arrest but could improve the efficiency of cell apoptosis due to the synergistic effect between these two components.
 | ||
| Fig. 3 Flow cytometry histogram of A549 cells treated with the release supernatants at 30 days from porous PLGA microparticles for 24 h: MP-1 (a), MP-2 (b), MP-3 (c) and MP-4 (d). | ||
 | ||
| Fig. 5 Induction of cell cycle arrest in A549 cells treated with the release supernatants at 30 days from porous PLGA microparticles for 24 h: control (a), MP-1 (b), MP-2 (c), MP-3 (d) and MP-4 (e). | ||
Finally, wound healing and Transwell migration assay were employed to determine whether the porous PLGA microparticles could inhibit the cell migration. Compared to the control and MP-1 group, porous microparticles loaded with doxorubicin and/or miR-34a (MP-2, MP-3 and MP-4) could obviously inhibit the cell migration, and MP-4 showed the highest inhibitory effect due to synergistic effect between these two components (Fig. S8 and S9†). Notably, wound size increased after 36 h in MP-2 and MP-4 treatment groups, which was probably caused by the activation of anti-proliferation and anti-migration pathway of miR-34a. Furthermore, Transwell migration assay was employed to detect the inhibition of cell migration, in which the representative images of migrated cells at the bottom of membrane were stained with crystal violet (Fig. 6). Compared to control or MP-1 group, the number of migrated cells was much lower in the cells treated with the release supernatants from porous PLGA microparticles MP-2, MP-3 and MP-4. As anticipated, MP-4 group showed the highest inhibition effect, much stronger than MP-2 and MP-3 groups. Thus, the co-delivery of doxorubicin and miR-34a via porous microparticles could successfully suppress the cell migration, which would be potential for solving the metastasis-related recurrence of lung cancers.
 | ||
| Fig. 6 Inhibition of cell migration in A549 cells treated with the release supernatants at 30 days from porous PLGA microparticles for 48 h: control (a), MP-1 (b), MP-2 (c), MP-3 (d) and MP-4 (e). | ||
In summary, porous PLGA microparticles for the co-delivery of chemotherapeutics and tumor suppressor miRNA were successfully constructed. The microparticles possessed highly porous surface, high encapsulation efficiency and favorable release profile. The synergistic effect between these two components ensured the efficient anti-proliferative and anti-migration effects of porous PLGA microparticles on tumor cells. Thus, the porous PLGA microparticles combining chemotherapy and gene therapy could solve the most important problems of malignant tumors, and are potential to be used as a sustained release system for lung cancer treatment via pulmonary administration.
Acknowledgements
The research was supported by Natural Science Foundation of China (no. 21204025, 81473142 and 81373344), the Ministry of Science and Technology of China (International Cooperation and Communication Program 2011DFR51090), the grants from Science & Technology Department of Jilin Province (no. 20130522005JH and 20140101140JC), and the Fundamental Research Funds for the Central Universities (JCKY-QKJC30).Notes and references
- D. H. Johnson, Clin. Lung Cancer, 1999, 1, 34 CrossRef CAS.
- X. Guo, X. Zhang, L. Ye, Y. Zhang, R. Ding, Y. Hao, Y. Zhao, Z. Zhang and Y. Zhang, J. Drug Targeting, 2012, 22, 421 CrossRef PubMed.
- H. A. Burris, Oncogene, 2009, 28, 4 CrossRef PubMed.
- S. L. Ginn, I. E. Alexander, M. L. Edelstein, M. R. Abedi and J. Wixon, J. Gene Med., 2013, 15, 65 CrossRef CAS PubMed.
- W. Sun, Y. S. Julie Li, H. D. Huang, J. Y. Shyy and S. Chien, Annu. Rev. Biomed. Eng., 2010, 12, 1 CrossRef CAS PubMed.
- D. P. Bartel, Cell, 2004, 116, 281 CrossRef CAS.
- P. Fasanaro, S. Greco, M. Ivan, M. C. Capogrossi and F. Martelli, Pharmacol. Ther., 2010, 125, 92 CrossRef CAS PubMed.
- A. G. Bader, D. Brown and M. Winkler, Cancer Res., 2010, 70, 7027 CrossRef CAS PubMed.
- Y. Chen, X. Zhu, X. Zhang, B. Liu and L. Huang, Mol. Ther., 2010, 18, 1650 CrossRef CAS PubMed.
- S. Shi, L. Han, T. Gong, Z. Zhang and X. Sun, Angew. Chem., Int. Ed., 2013, 52, 3901 CrossRef CAS PubMed.
- X. Wu, H. Chen and X. Wang, Cancer Treat. Rev., 2012, 38, 580 CrossRef PubMed.
- H. Hermeking, Cell Death Differ., 2009, 17, 193 CrossRef PubMed.
- X. Deng, M. Cao, J. Zhang, K. Hua, Z. Yin, Z. Zhou, X. Xiao, Y. Yang, W. Sheng, Y. Wu and Y. Zeng, Biomaterials, 2014, 35, 4333 CrossRef CAS PubMed.
- S. K. Kim, M. B. Foote and L. Huang, Biomaterials, 2012, 33, 3959 CrossRef CAS PubMed.
- J. S. Patton and P. R. Byron, Nat. Rev. Drug Discovery, 2007, 6, 67 CrossRef CAS PubMed.
- K. Hirota, Y. Oishi, H. Taniguchi, K. Sawachi, H. Inagawa, C. Kohchi, G. Soma and H. Terada, Anticancer Res., 2010, 30, 3129 CAS.
- O. B. Garbuzenko, M. Saad, V. P. Pozharov, K. R. Reuhl, G. Mainelis and T. Minko, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 10737 CrossRef CAS PubMed.
- D. A. Edwards, J. Hanes, G. Caponetti, J. Hrkach, A. Ben-Jebria, M. L. Eskew, J. Mintzes, D. Deaver, N. Lotan and R. Langer, Science, 1997, 276, 1868 CrossRef CAS.
- T. K. Kim, J. J. Yoon, D. S. Lee and T. G. Park, Biomaterials, 2006, 27, 152 CrossRef CAS PubMed.
- M. J. Kwon, J. H. Bae, J. J. Kim, K. Na and E. S. Lee, Int. J. Pharm., 2007, 335, 5 CrossRef PubMed.
- Y. Yang, N. Bajaj, P. Xu, K. Ohn, M. D. Tsifansky and Y. Yeo, Biomaterials, 2009, 30, 1947 CrossRef CAS PubMed.
- Y. Cai, Y. Chen, X. Hong, Z. Liu and W. Yuan, Int. J. Nanomed., 2013, 8, 1111 Search PubMed.
- K. Koushik and U. B. Kompella, Pharm. Res., 2004, 21, 524 CrossRef CAS.
- K. Koushik, D. S. Dhanda, N. P. S. Cheruvu and U. B. Kompella, Pharm. Res., 2004, 11, 1119 CrossRef.
- J. Liu, D. Meisner, E. Kwong, X. Y. Wu and M. R. Johnston, Biomaterials, 2007, 28, 3236 CrossRef CAS PubMed.
- H. Kim, J. Lee, T. H. Kim, E. S. Lee, K. T. Oh, D. H. Lee, E. S. Park, Y. H. Bae, K. C. Lee and Y. S. Youn, Pharm. Res., 2011, 28, 2008 CrossRef CAS PubMed.
- T. Feng, H. Tian, C. Xu, L. Lin, Z. Xie, M. H. Lam, H. Liang and X. Chen, Eur. J. Pharm. Biopharm., 2014, 88, 1086 CrossRef CAS PubMed.
- X. Shi, C. Li, S. Gao, L. Zhang, H. Han, J. Zhang, W. Shi and Q. Li, Colloids Surf., B, 2014, 122, 498 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15516a |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2015 |

