Acid-amplifying polymers: synthesis, characterization, and application to environmentally stable chemical amplification positive (ESCAP) resists†
K. Arimitsu*,
M. Yonekura and
M. Furutani
Department of Pure and Applied Chemistry, Tokyo University of Science, 2641 Yamazaki, Noda, Chiba 278-8510, Japan. E-mail: arimitsu@rs.noda.tus.ac.jp; Fax: +81-4-7123-9890; Tel: +81-4-7124-1501
First published on 14th September 2015
Abstract
Acid amplifiers that are decomposed autocatalytically by acid species photogenerated from photoacid generators were synthesized and characterized. Such acid proliferation reactions by acid amplifiers were integrated into chemical amplification resists to improve the photosensitivity. In this study, new polymers bearing acid-amplifying (AA) units in their side chains were designed to suppress excess diffusion of acid species, and then evaluated for their photosensitivity with photoacid generators as environmentally stable chemical amplification positive (ESCAP) resists. The AA polymers have high photosensitivity (up to 1.4 mJ cm−2) after 254 nm of light irradiation and post-exposure baking at 140 °C. Using films of these polymers, 1 × 4 μm line-and-space patterns were fabricated.
Introduction
Photoreactive materials are used in many industrial fields, such as electronics, car manufacturing, construction, and adhesives. UV light sources are often used. These materials generally consist of a reactive polymer and a photoinitiator. One of the most common combinations is an acid-reactive polymer with a photoacid generator (PAG).1–5 PAGs photodecompose to generate acid species that catalyse the polymer reactions, resulting in significant changes in the solubility of the polymers. The photosensitivity of such a system could be higher than that of a system that only includes a photoreactive polymer. The former is often referred to as chemical amplification.6For further improvement of the photosensitivity of such photoreactive materials, we propose the use of acid amplifiers that are autocatalytically decomposed by photogenerated acid species from PAGs.7 Acid proliferation reactions by acid amplifiers make it possible to increase, non-linearly, the amount of acid catalysts in a system. The reaction system has been applied to photoresists for improving their photosensitivity.8,9 However, the acid species from acid amplifiers often have low molecular weights, and they are prone to excessive diffusion in air or the polymer matrix, leading to reduced resolution.7b,10 To overcome this problem, acid-reactive polymers bearing acid-amplifying (AA) units have been proposed.11 The application of these polymers to photoresists is limited, however, because the polymer, after acid-catalysed decomposition, is not developable with alkaline solution. In this study, AA units were integrated into a polymer having 4-hydroxystyrene and tert-butyl acrylate units to give environmentally stable chemical amplification positive (ESCAP) resists (Scheme 1). These resists have been applied to photolithography using a KrF excimer (248 nm) laser.12 The acid species are generated in a non-linear manner at their polymer chains, which reduces the diffusion of acid species. The suppression effect (suppression of the excess diffusion of acid species) achieved by the integration, and the improvement of the photosensitivity as a novel ESCAP resist were investigated using 254 nm light. Fig. 1 shows a conventional low molecular weight acid amplifier 3 for comparison with the polymers tethering AA units and PAGs used in this study. The polymers were used to fabricate micro-patterns with high photosensitivity.
 | ||
| Scheme 1 Phototriggered acid proliferation reaction system used in this study, and chemical structures of copolymers 1 and 2. | ||
Experimental
Materials
The reagents used were purchased from Tokyo Chemical Industry Co. (Tokyo, Japan) and Wako Pure Chemical Industries (Osaka, Japan). PAGs used in this study were provided by Midori Kagaku Co., Ltd (Tokyo, Japan). All chemicals were used without further purification.Analysis
1H NMR spectra were obtained using a Jeol JNM-ECP500 or JNM-ECP300 spectrometer (Jeol, Japan). FT-IR spectra were measured using a Jasco FT/IR-6100 spectrophotometer (Jasco, Japan). GPC measurements were performed using a Hitachi L-2490 detector and a Hitachi Gelpack GL-A140-S column, and the molecular weight data were obtained relative to polystyrene standards. Photoirradiation was carried out using a San-Ei Electric Hg–Xe lamp, or a Funakoshi UVG-11 lamp. The quantity of light was measured using an Advantest TQ8210. Film thickness was measured using a Dektak150 (Ulvac, Japan). Line & space patterns were observed using a Keyence VK8500 laser microscope (Keyence, Japan).Syntheses of acid-amplifying monomers and acid amplifiers
The AA monomer was then synthesized from 4-styrenesulfonyl chloride and (1S,2S,3R,5S)-(+)-2,3-pinanediol, according to a method described in the literature.11 It was obtained as a pale yellow viscous liquid in 76% yield (3.03 g). Identification was performed using 1H NMR (300 MHz, CDCl3): δ 0.9 (3H, s, CH3C(OH)<), 1.2–1.3 (6H, s + s, >(CH3)2), 1.5–2.5 (7H, m, methylene, methine, –OH), 4.9 (1H, m, –SO3CH<), 5.5 (1H, d, J = 12 Hz, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 5.9 (1H, d, J = 18 Hz, CH2
CH–), 5.9 (1H, d, J = 18 Hz, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 6.8 (1H, dd, J = 12, 18 Hz, CH2
CH–), 6.8 (1H, dd, J = 12, 18 Hz, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–), 7.6–7.9 (4H, m, aromatic).
CH–), 7.6–7.9 (4H, m, aromatic).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–), 6.0 (1H, m, CH2
C(CH3)–), 6.0 (1H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–).
C(CH3)–).3-Chlorosulfonylpropyl methacrylate (1.10 g, 4.8 mmol) in DCM (5 mL) was added dropwise to a solution of (1S,2S,3R,5S)-(+)-2,3-pinanediol (0.5 g, 2.9 mmol), 4-dimethylaminopyridine (DMAP) (0.14 g, 1.1 mmol) and triethylamine (0.8 g, 7.9 mmol) at 0 °C. The reaction mixture was stirred at room temperature for 2 days. After washing with 5 wt% aqueous HCl solution, saturated with aqueous NaHCO3 solution and brine, the organic layer was dried over MgSO4. Column chromatography was performed with hexane/ethyl acetate (4/1) to afford the AA monomer as a pale yellow viscous liquid in 88% yield (0.94 g). 1H NMR (500 MHz, CDCl3): δ 1.0 (3H, s, CH3C(OH)<), 1.3 (3H, s, >(CH3)2), 1.4 (3H, s, >(CH3)2), 1.5–2.6 (7H, m, methylene, methine, –OH), 3.3 (2H, m, –CH2–SO3–), 4.3 (2H, m, –CO2CH2–), 5.0 (1H, m, –SO3CH<), 5.6 (1H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–), 6.1 (1H, m, CH2
C(CH3)–), 6.1 (1H, m, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)–). 13C NMR (125 MHz, CDCl3): δ 18, 23, 24, 28, 28, 29, 35, 38, 40, 48, 54, 62, 79, 126, 136, 167. FT-IR (neat, cm−1): 941 (S–O–C), 1329 (S
C(CH3)–). 13C NMR (125 MHz, CDCl3): δ 18, 23, 24, 28, 28, 29, 35, 38, 40, 48, 54, 62, 79, 126, 136, 167. FT-IR (neat, cm−1): 941 (S–O–C), 1329 (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1719 (C
O), 1719 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 3538 (OH). HR-MS. Found: 361.1691. Calc for C17H29SO6 ([M + H]+): 361.1685.
O), 3538 (OH). HR-MS. Found: 361.1691. Calc for C17H29SO6 ([M + H]+): 361.1685.
General procedure of copolymerization
Copolymerization using the AA monomers was performed according to a literature procedure.12 Briefly, 4-acetoxystyrene (AS), tert-butyl methacrylate (tBMA), AA monomer and 2,2′-azobis-isobutyronitrile (AIBN) were mixed in THF, and sparged with N2 gas for 30 min. The solution was boiled under reflux for 12 h, and then reprecipitated three times with hexane as a poor solvent. After vacuum drying, the residue was dissolved in methanol/THF (1/1). A 28% aqueous ammonia solution was added, and the resulting solution was stirred at room temperature for 6 h. After evaporation, the residue was dissolved in acetone, which was then reprecipitated three times with water as a poor solvent. Vacuum drying afforded the product as a white solid. Copolymerization ratios were estimated using 1H NMR analysis.As a control, a copolymer without AA units was also synthesized (denoted as ESCAP in this paper). Using AS (0.74 g, 4.6 mmol) and tBMA (2.6 g, 18 mmol) as monomers with AIBN (0.44 g), ESCAP was obtained (1.9 g, l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n = 25
n = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, determined by 1H NMR, Mn = 0.57 × 104, Mw/Mn = 1.5).
0, determined by 1H NMR, Mn = 0.57 × 104, Mw/Mn = 1.5).
Acid-catalysed decomposition behaviour
Copolymer 1c (0.1 g) and 4-toluenesulfonic acid (TsOH, 0 or 1 wt%) were dissolved in THF (0.8 mL). The solution was spin cast on a Si wafer (1000 rpm, 30 s), which was prebaked at 100 °C for 1 min. The film thickness was 4.7 μm. FT-IR spectral measurement was performed with heating at 120 °C for 0–200 min.Evaluation of diffusion behaviour of acid species
Copolymer 1c and PAG 4 (1 wt%) were dissolved in propylene glycol monomethyl ether acetate (PGMEA, 0.9 g). As a control, a PGMEA solution consisting of ESCAP and acid amplifier 3 (cis-3-[4-toluenesulfonyloxy]-2-pinanol, 20 wt%) was also prepared. Each solution was spin cast on a Si wafer (1000 rpm, 30 s), which was prebaked at 100 °C for 1 min. The film thickness was ca. 0.4 μm. UV irradiation was performed using a Hg–Xe lamp, through a photomask with a circular hole 3 mm in diameter. Postbaking was performed at 120 °C. Thereafter, the cross-sectional shape was observed and investigated.Sensitivity evaluation
Copolymers 1a, 2a–c, or ESCAP (0.1 g) and PAG 5 (1 wt%) were dissolved in PGMEA (0.8–0.9 g). Each solution was spin cast on a Si wafer (1000 or 3000 rpm, 30 s), which was prebaked at 100 °C for 1 min. The film thicknesses were all ca. 0.4 μm. After 254 nm light irradiation and postbaking, the films were developed with a 2.38 wt% aqueous solution of tetramethylammonium hydroxide (TMAH) for 30 s, followed by rinsing in water. The film thicknesses were measured.Photopatterning
Copolymer 2a and PAG 5 (1 wt%) were dissolved in PGMEA (0.9 g). The solution was spin cast on a Si wafer (1000 rpm, 30 s), which was prebaked at 100 °C for 1 min. The film thickness was 0.3–0.4 μm. After 254 nm light irradiation (4 mJ cm−2), through a photomask, and postbaking at 140 °C for 110 s, the film was developed with 2.38 wt% aqueous TMAH solution for 60 s, followed by rinsing in water. The resulting patterns were observed using a laser microscope.Results and discussion
Syntheses of copolymers bearing AA units
Copolymers 1a–c containing AA units were synthesized, as shown in Table 1. Three types of monomers, AS, tBMA and AA1′, were randomly copolymerized, resulting in molecular weights of 6300–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The molar ratios of monomers were almost coincident with the ratios in the copolymers. AA units were introduced in the ratio of 3–10 mol%. Copolymers 2a–c containing other types of AA units were also synthesized, using monomers AS, tBMA and AA2′, as shown in Table 2. The molecular weights were 6000–7500, and the ratios of the AA units introduced were 2–10 mol%.
000. The molar ratios of monomers were almost coincident with the ratios in the copolymers. AA units were introduced in the ratio of 3–10 mol%. Copolymers 2a–c containing other types of AA units were also synthesized, using monomers AS, tBMA and AA2′, as shown in Table 2. The molecular weights were 6000–7500, and the ratios of the AA units introduced were 2–10 mol%.
| Run | Monomer [g (mmol)] | Molar ratio | AIBN [g] | Copolymer | Yield [g] | l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m : m :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n |
Mn [104] | Mw/Mn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AS | tBMA | AA1′ | ||||||||
| 1 | 0.79 (4.8) | 2.4 (17) | 0.20 (0.58) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.36 | 1a | 2.2 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.63 | 1.5 |
| 2 | 0.79 (4.8) | 2.4 (17) | 0.41 (1.2) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.38 | 1b | 2.3 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
1.2 | 1.7 |
| 3 | 0.78 (4.8) | 2.4 (17) | 0.78 (2.3) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.35 | 1c | 2.3 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1.0 | 1.7 |
| Run | Monomer [g (mmol)] | Molar ratio | AIBN [g] | Copolymer | Yield [g] | l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n |
Mn [104] | Mw/Mn | ||
|---|---|---|---|---|---|---|---|---|---|---|
| AS | tBMA | AA2′ | ||||||||
| 1 | 0.69 (4.3) | 2.1 (15) | 0.16 (0.43) | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.32 | 2a | 1.4 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.68 | 1.3 |
| 2 | 0.68 (4.2) | 2.1 (15) | 0.36 (1.0) | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
0.31 | 2b | 1.7 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 71 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.60 | 1.5 |
| 3 | 0.58 (3.6) | 1.8 (13) | 0.67 (1.8) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.30 | 2c | 1.9 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 68 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.75 | 1.6 |
Decomposition behaviour of copolymers bearing with AA units
Polymers bearing AA units should fulfil the following requirements.7 First, the polymer should be readily decomposed by acid catalysis to liberate the sulfo groups at their side chains. Second, the sulfo groups formed should be strong enough acids to catalyse the decomposition reaction of polymer side chains to form more sulfo groups, leading to autocatalytic decomposition. Third, the polymer should be thermally stable in the absence of acid, at least under the reaction conditions, so as to enhance the autocatalytic decomposition initiated by the photogenerated acid. The thermal stability and decomposition behaviour of copolymer 1c were estimated by plotting the amounts of the remaining sulfonate groups and the generating sulfo groups against time (Fig. 2). The intensity of the peak at 941 cm−1 assigned to sulfonate decreased, while the intensity of the peak at 1008 cm−1 assigned to sulfonic acid increased.14 This indicated that AA units of the copolymer had been decomposed. In the case of 1 wt% TsOH, the decomposition occurred rapidly upon heating. On the other hand, an induction period of a few minutes was observed in the case without TsOH. In both cases, sigmoidal reaction curves were recorded in the measurements, which means that acid proliferation reactions, autocatalytic decomposition reactions of 1c, proceed with a small amount of acid species as a trigger. A similar decomposition behaviour was also observed for a film of copolymer 2c (see ESI†).Diffusion behaviour of acid species
An ESCAP film containing a conventional acid amplifier 3 was fabricated. After UV irradiation, through a photomask with a circular hole of 3 mm in diameter, the colour of the exposed part clearly changed with postbaking at 120 °C for 2 min (Fig. 3(a)). This would be because of changes in film thickness, due to partial decomposition of tert-butyl ester moieties at the side chains of the copolymer caused by photogenerated acids from PAGs. A similar result was obtained in the case of a film of copolymer 1c (Fig. 3(b)).The discoloured part increased during postbaking at 120 °C in the former case (ESCAP/3, Fig. 3(c)). When the acid proliferation reaction proceeded with acid amplifiers, the generated acid species would diffuse excessively to the unexposed part. On the other hand, in the latter case (1c), the discoloured part of the film did not increase at all (Fig. 3(d)). Copolymer 1c would have acidic units at its side chains after the acid proliferation reaction, resulting in an effective suppression of the excess diffusion of acid species. The diameters of the discoloured circles during postbaking were plotted against the heating time, as shown in Fig. 3(e). In the former case (ESCAP/3), the diameter increased to >10 mm after 12 min. In the latter case (1c), however, it remained at 3 mm after 20 min. Thus, acid proliferation reactions without excess diffusion of acid species were achieved.
Depth profiles also show clearly the difference in the diffusion behaviour between these films (Fig. 4). In the case of the ESCAP film containing acid amplifier 3, it was found that deformation occurred in the upper region of the film in particular, indicating ‘air infection’ due to a low molecular weight of acid species, as shown by comparing the profiles in Fig. 4(a) and (c).7b,10 Acid species would be diffused on the film by repeated volatilization and attachment, which would cause unnecessary acid proliferation reactions in the unexposed region. On the other hand, in the case of a film of copolymer 1c, the width was almost constant, even after postbaking (Fig. 4(b) and (d)). Acid proliferation reactions between AA units would lead to the generation of acid species with high molecular weights, which would prevent such an ‘infection’ phenomenon.
 | ||
| Fig. 4 Depth profiles of colour-changed circular areas after UV irradiation and subsequent heating at 120 °C, for 2 min (a) ESCAP + 3, (b) 1c; or 8 min (c) ESCAP + 3, (d) 1c. | ||
Photosensitivity determination
Copolymers 1 and 2 were catalytically decomposed when carboxy and sulfo groups were generated through acid-catalysed and acid proliferation reactions, respectively. These reactions result in the copolymers being soluble in polar developers such as aqueous TMAH. Photosensitivity determination was performed using films consisting of copolymers 1a, 2a, or ESCAP, with 254 nm light irradiation and postbaking at 140 °C for 2 min.15 Development was performed with 2.38 wt% aqueous TMAH. Normalized film thickness was plotted against the exposure dose, as shown in Fig. 5. A control film consisting of ESCAP and 1 wt% PAG 5 was not fully dissolved, even with 320 mJ cm−2 of irradiation energy. The deprotection reaction of tert-butyl groups did not proceed adequately with photogenerated acids. On the other hand, 15 mJ cm−2 of photosensitivity was accomplished using copolymer 1a with the same amount of PAG.16 AA units of 1a were decomposed autocatalytically by photogenerated acids, leading to an efficient deprotection reaction. It is noteworthy that acid proliferation reactions have the potential to significantly improve the photosensitivity of ESCAP resists. Furthermore, when using copolymer 2a instead of 1a, only 1.4 mJ cm−2 of irradiation energy was required for complete solubilization. After adding 1 wt% TsOH to films of 1a or 2a, the decomposition behaviour was evaluated by FT-IR spectral measurements.17 It was found that AA units of 2a decomposed more quickly than did those of 1a (see ESI†). Copolymer 2a may have more flexible sulfonic acid units at its side chains than 1a, which would lead to an efficient acid proliferation reaction. | ||
| Fig. 5 Photosensitivity curves of films of copolymer 1a, 2a and ESCAP containing 1 wt% of PAG 5, with 254 nm light irradiation and postbaking at 140 °C for 2 min. | ||
It was also found that the number of AA units in the copolymer influences the photosensitivity (Fig. 6). Postbaking was performed at 130 °C for 3 min, and the values of sensitivity were 1.8 (2c, 10 mol% of AA units), 6.0 (2b, 6 mol%) and 13 mJ cm−2 (2a, 2 mol%), respectively. It was clear that the density of AA units in an ESCAP resist film contributes to the efficiency of the acid proliferation reaction.
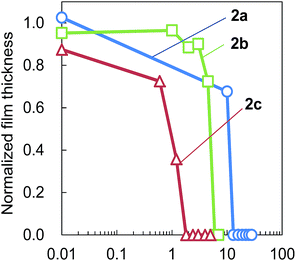 | ||
| Fig. 6 Photosensitivity curves of films of copolymer 2 resists containing 5 wt% of PAG 5, with 254 nm light irradiation and postbaking at 130 °C for 3 min. | ||
Photopatterning
Micro-scale patterns were fabricated using a film of copolymer 2a containing 1 wt% PAG 5. Irradiation energy of 254 nm light was just 4 mJ cm−2, and postbaking was performed at 140 °C for 110 s. Fig. 7 shows that positive-tone patterns having 10 × 10, 5 × 4, 3 × 4, and 1 × 4 μm line-and-space features were successfully obtained. | ||
| Fig. 7 (a) Positive-tone patterned resist films of copolymer 2a, with 4 mJ cm−2 of 254 nm light irradiation and postbaking at 140 °C for 110 s. (b) Photomasks used in this experiment. | ||
Conclusions
Copolymers 1 and 2 having 2–10 mol% of AA units were designed and synthesized for an ESCAP resist system. Using these copolymers, we confirmed that an acid proliferation reaction proceeds, and that excess diffusion of the acid species is effectively prevented in their films. This is not accomplished with films containing conventional low molecular weight acid amplifiers. Compared with an ESCAP/PAG system, the 2 (or 1)/PAG system showed much higher photosensitivity: the maximum value was 1.4 mJ cm−2. It was determined that the acid proliferation reaction is quite effective for improving the photosensitivity of ESCAP films. Copolymer 2a was applied to a positive-working patterning process, and line-and-space patterns ranging from 10 × 10 to 1 × 4 μm were obtained with 254 nm light irradiation and postbaking at 140 °C. ESCAP resists could be applied to extreme UV photolithography systems. However, to apply the copolymers considered in this study for such advanced systems, further investigations should be carried out.Notes and references
- S. A. McDonald, C. G. Willson and J. M. J. Fréchet, Acc. Chem. Res., 1994, 27, 151 CrossRef.
- H. Ito, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 3863 CrossRef CAS PubMed.
- T. Aoai, Y. Aotani, A. Umehara and T. Kokubo, J. Photopolym. Sci. Technol., 1990, 3, 389 CrossRef CAS.
- M. Sakata, T. Ito and Y. Yamashita, Jpn. J. Appl. Phys., Part 1, 1991, 30, 3116 CrossRef CAS.
- T. Ito, M. Sakata, A. Endo, H. Jinbo and I. Ashida, Jpn. J. Appl. Phys., Part 1, 1993, 32, 6052 CrossRef CAS.
- H. Ito and C. G. Willson, Polym. Eng. Sci., 1983, 23, 1012 CAS.
- (a) K. Ichimura, K. Arimitsu and K. Kudo, Chem. Lett., 1995, 551 CrossRef CAS; (b) K. Arimitsu, K. Kudo and K. Ichimura, J. Am. Chem. Soc., 1998, 120, 37 CrossRef CAS.
- (a) T. Ohfuji, M. Takahashi, M. Sasago, S. Noguchi and K. Ichimura, J. Photopolym. Sci. Technol., 1997, 10, 551 CrossRef CAS; (b) K. Kudo, K. Arimitsu, H. Ohmori, H. Ito and K. Ichimura, Chem. Mater., 1999, 11, 2119 CrossRef CAS; (c) S.-W. Park, K. Arimitsu, K. Ichimura and T. Ohfuji, J. Photopolym. Sci. Technol., 1999, 12, 293 CrossRef CAS; (d) T. Naito, T. Ohfuji, M. Endo, H. Morimoto, K. Arimitsu and K. Ichimura, J. Photopolym. Sci. Technol., 1999, 12, 509 CrossRef CAS; (e) K. Arimitsu, K. Kudo, H. Ohmori and K. Ichimura, J. Mater. Chem., 2001, 11, 295 RSC; (f) S.-W. Park, K. Arimitsu and K. Ichimura, Macromol. Rapid Commun., 2000, 21, 1050 CrossRef CAS; (g) S. Lee, K. Arimitsu, S.-W. Park and K. Ichimura, J. Photopolym. Sci. Technol., 2000, 13, 215 CrossRef CAS; (h) K. Arimitsu and K. Ichimura, Chem. Lett., 1998, 823 CrossRef CAS; (i) H. Ito and K. Ichimura, Macromol. Chem. Phys., 2000, 201, 132 CrossRef CAS.
- K. Hosoi, B. Cardineau, S. Kruger, K. Miyauchi and R. Brainard, J. Photopolym. Sci. Technol., 2012, 25, 575 CrossRef CAS.
- K. Kudo, K. Arimitsu, H. Ohmori, H. Ito and K. Ichimura, Chem. Mater., 1999, 11, 2126 CrossRef CAS.
- S.-W. Park, K. Arimitsu and K. Ichimura, J. Photopolym. Sci. Technol., 2004, 17, 427 CrossRef CAS.
- H. Ito, G. Breyta, D. Hofer and R. Sooriyakumaran, J. Photopolym. Sci. Technol., 1994, 7, 433 CrossRef CAS.
- M. Tada, R. Coquet, J. Yoshida, M. Kinoshita and Y. Iwasawa, Angew. Chem., Int. Ed., 2007, 46, 7220 CrossRef CAS PubMed.
- M. Shirai, A. Kawaue, H. Okamura and M. Tsunooka, Chem. Mater., 2003, 15, 4075 CrossRef CAS.
- Postbaking temperature and time examined at 120–140 °C for 1–3 min. The results are shown in ESI.†.
- Photosensitivity is defined as the irradiation energy required to completely dissolve the film (normalized film thickness becomes zero).
- The films were prepared according to the procedure described in the Experimental section (see ‘Acid-catalysed decomposition behaviour’). PGMEA was used instead of THF.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15423e |
| This journal is © The Royal Society of Chemistry 2015 |



