A turn-on fluorescent probe for hydrogen sulfide and its application in living cells†
Abstract
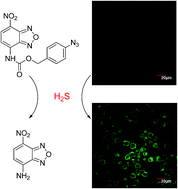
Hydrogen sulfide (H2S) is an important endogenous signalling molecule in cellular physiology and pathology. Accordingly, sensitive, selective and reliable methods for H2S detection are in high demand. Fluorescence-based methods thus have received great attentions in recent years. Herein we describe the design, synthesis and application of a probe for H2S detection based on the reduction of azide to amine. This probe is highly sensitive and selective toward H2S over biothiols and other biologically relevant species. Finally, the probe was successfully applied for H2S fluorescent imaging in living cells.

 Please wait while we load your content...
Please wait while we load your content...