Regulating the hydrothermal synthesis of ZnO nanorods to optimize the performance of spirally hierarchical structure-based glucose sensors†
W. X. Jing*a,
F. Zhoua,
W. Z. Gaoa,
Z. D. Jianga,
W. Renb,
J. F. Shia,
Y. Y. Chenga and
K. Gaoa
aState Key Laboratory for Manufacturing Systems Engineering at Xi'an Jiaotong University, Xi'an 710049, China. E-mail: wxjing@mail.xjtu.edu.cn
bElectronic Materials Research Laboratory, Key Laboratory of the Ministry of Education, International Center for Dielectric Research, Xi'an Jiaotong University, Xi'an 710049, China
First published on 5th October 2015
Abstract
This paper reports the effects of the synthesizing parameters on the surface morphologies of ZnO nanorod-based spirally hierarchical structures and the performance of related spirally hierarchical structure-based glucose sensors. ZnO nanorods were hydrothermally synthesized on Au cylindrical spirals with 3 sets of the synthesizing parameters, and glucose oxidase (GOx) was immobilized on these ZnO nanorods, thus 3 batches of the spirally hierarchical structure-based glucose enzymatic electrodes were fabricated. Geometric, crystalline and electrochemical characterization indicates that of all 3 batches of the spirally hierarchical structures, those fabricated respectively at 25 mM Zn2+ concentration of the growth solution, for 1.5 h the growth duration, and at 0.5 mM Zn2+ concentration of the seed solution all have Gaussian random rough surfaces. This gives rise to the largest surface area of the related spirally hierarchical structure, the most effective GOx immobilization of the corresponding enzymatic electrode, and the optimal performance of the related glucose sensor. The results benefit not only the batch construction but also the standardization of other hierarchical structure-based glucose sensors.
1. Introduction
A variety of hierarchical structures with larger surface areas are widely used to construct the enzymatic electrodes of different electrochemical glucose sensors in medicine, biology, food industry and environmental monitoring domains.1–4 Construction of a hierarchical structure often includes fabrication of the micro-scale substrate and synthesis of the nano-scale matrix material. For instance, fibres of Au, Ag, and Pt are often employed as cylindrical substrates,5–7 indium tin oxide (ITO) glass,8 fluorine-doped tin oxide (FTO) glass,9 and Au-coated Si and glass10,11 as planar substrates, and Au microspheres as spherical substrates.12 Nevertheless, the fibres and microspheres are with thinner diameters and thus quite soft, so it is exceedingly difficult to manipulate these micro-scale substrates. In addition, the planar substrates readily result in enzyme accumulation and thus lower the performance of the related glucose sensors. Moreover aforementioned cylindrical, planar and spherical micro-scale substrates have either longer length or larger volumes, which hinders the miniaturization of the related enzymatic electrodes. Therefore a novel micro-scale substrate with compact size, larger surface area and readily manipulation needs to be put forward. ZnO nanorods have larger specific surface area, higher isoelectric point, strong resistance to be oxidized, and excellent biocompatibility, and are frequently used as ideal matrix material for the construction of various hierarchical structure-based glucose sensors.13–15 In comparison with chemical vapor deposition (CVD),16 radio frequency (RF) magnetron sputtering17 and electro-deposition,18 hydrothermal synthesis19 is not only simple and cost-effective but also leads to ZnO nanorods of high quality, so it is used in this paper.The hydrothermal synthesizing parameters of ZnO nanorods include Zn2+ concentration of growth and seed solution, growth duration, and growth temperature. They greatly affect the diameters, lengths, and direction of ZnO nanorods on a micro-scale substrate,20,21 which further results in different GOx immobilization on these ZnO nanorods,22 and eventually the performance of the related hierarchical structure-based glucose sensor.23 As a consequence these synthesizing parameters should be finely regulated. For instance, Ahmad22 changed the growth duration to synthesize the ZnO nanorods of different aspect ratios on the planar Ag-coated Si substrates, and established the brief relationship between this synthesizing parameter and the performance of the glucose sensors. Kim23 modified Zn2+ concentration of the both growth and seed solution to synthesize the ZnO nanorods of different diameters on the planar Au-coated glass electrodes, and found that the larger the diameters of the ZnO nanorods are, the better the performances of the glucose sensors. Zhai24 regulated Zn2+ concentration of the precursor, electrolytes and deposition charges to electrochemically deposit the ZnO nanorods/nanoplates of different morphologies on the ITO substrates, and discussed the effects of the size, shape and deposition density of the ZnO nanomaterials on the performance of the glucose sensors. As a matter of fact, not only the diameters, lengths and direction of ZnO nanorods but also the sizes of pits among the bundles of ZnO nanorods all impact significantly GOx immobilization on ZnO nanorod-based hierarchical structures. Nonetheless, it is difficult to quantitatively describe the sizes of the pits which interrelate with the diameters and direction of ZnO nanorods. To our knowledge this topic has not been investigated in literatures yet. Since the surface morphology of a ZnO nanorod-based hierarchical structure is attributed to the combined effects of the aforementioned influencing factors, once the surface morphology is statistically characterized, the relationship between the synthesizing parameters, the surface morphology of the hierarchical structure, the GOx immobilization on the enzymatic electrode, and the performance of the glucose sensor can be built.25 Furthermore with this relationship established, the synthesizing parameters can be optimized, the batch fabrication process be standardized, and more importantly, the performance of the glucose sensors be improved.
Direct measurement with a scanning electron microscopy (SEM) is frequently used to determine the geometric parameters of various ZnO nanorod-based hierarchical structures and related enzymatic electrodes.26 For example, with SEM micrographs and related scale bars Kim23 and Usman Ali27 acquired the diameters and lengths of the ZnO nanorods hydrothermally synthesized on the planar Au-coated glass and plastic substrates respectively. Similarly Zhong28 derived the diameters and lengths of the ZnO nanorods/nanowires synthesized by CVD on the flexible graphite sheets, and qualitatively characterized the morphology of the ZnO nanostructures. Obviously the introduced characterization parameters are sparse, and the measurement data insufficient. Additionally, since the line edges of the features of interest are not extracted from related SEM micrographs, it is impossible to quantitatively characterize the surface morphologies of the ZnO nanorod-based hierarchical structures. These disadvantages further give rise to incomplete geometric evaluation of the hierarchical structures, and hinder optimization of the fabrication processes and quality improvement of the enzymatic electrodes.
This paper reports the batch construction and quantitative characterization of ZnO nanorod-based spirally hierarchical structures and related enzymatic electrodes. The effects of synthesizing parameters on the surface morphologies, the GOx immobilization, and the performance of the spirally hierarchical structure-based glucose sensors were investigated.
2. Experimental
2.1 Materials and apparatus
Multimode optical fibres (nominal diameter of the core 125 μm) and Au fibres (nominal diameter 30 μm) were purchased from Quanzhou Anpon Company and Beijing Doublink Solders Co., Ltd respectively. D-(+)-Glucose (purity 99.9%) was bought from Sigma. Glucose oxidase (lyophilized powder, activity > 180 U mg−1) was supplied by Aladdin. Acetone, AR absolute ethyl alcohol, and zinc acetate dihydrate (Zn(CH3COO)2·2H2O) were bought from Tianjin Kemiou Chemical Reagent Co., Ltd. Zinc nitrate (Zn(NO3)2·6H2O), sodium hydrate (NaOH), hexamethylenetetramine (C6H12N4) were purchased from Tianjin Fuchen Chemical Reagent Factory. Epoxy resin was obtained from Zibo Huadong Yuhua Industry & Trade Co., Ltd. Phosphate buffered solution (PBS, pH 7.4) of 10 mM was prepared with certain amount of Na2HPO4·12H2O and KH2PO4 in de-ionized (DI) water. Glucose stock solution was kept for at least 24 h for mutarotation before use. All other chemicals were of analytical grade.With a SU-8010 SEM (Hitachi, Japan) and R2008a MATLAB software (MathWorks Company) the geometric parameters of the Au cylindrical spirals and spirally hierarchical structures were quantitatively characterized. The crystal structures of the ZnO nanorods were examined by X-ray diffractometer (XRD, Panak). Further structure analysis of an individual ZnO nanorod was carried out in a JEM 2100F transmission electron microscopy (TEM, JEOL) with selected area electron diffraction (SAED) analysis. Cyclic voltammetry and amperometric response were performed on a CHI660D electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd). A conventional three-electrode system was employed which contains the proposed enzymatic electrode as the working electrode, an Ag/AgCl electrode as the reference electrode, and a Pt wire as the counter electrode.
2.2 Fabrication of ZnO nanorod-based spirally hierarchical structures
The fabrication of a ZnO nanorod-based spirally hierarchical structure includes construction of a micro-scale Au cylindrical spiral and hydrothermal synthesis of ZnO nanorods. A multimode optical fibre was immersed in acetone for 5 min, and the optical fibre core was pulled out from its sheath. One end of an Au fibre was fixed with epoxy resin on one end face of the optical fibre core. Then the Au fibre was manually spiralled compactly around the optical fibre core till the axial length of the Au cylindrical spiral reached to 3 mm. The other end of this Au cylindrical spiral was securely fixed with epoxy resin on the cylindrical surface of the optical fibre core. The assembly was cleaned in absolute ethyl alcohol in an ultrasonic washer and dried in a vacuum drier, thus an Au cylindrical spiral was formed.The seed solution of different Zn2+ concentration was prepared with Zn(CH3COO)2·2H2O, NaOH, and absolute ethyl alcohol,29 whilst the growth solution of various Zn2+ concentration was produced with Zn(NO3)2·6H2O, C6H12N4 and DI water.30 Table 1 illustrates 3 sets of the synthesizing parameters. Six Au cylindrical spirals were immersed into the seed solution of 1 mM Zn2+ concentration for 1 min, and then annealed at 150 °C for 10 min. These immersing and annealing processes were repeated twice more. The ZnO seed-coated Au cylindrical spirals were put into the growth solution of different Zn2+ concentration respectively at 90 °C for 2.5 h, then taken out, cleaned with DI water for 5 min in the ultrasonic washer, and dried at room temperature. Therefore the 1st batch of the spirally hierarchical structures was fabricated. Similarly with varied growth durations and different Zn2+ concentration of the seed solution, the 2nd and 3rd batches of the spirally hierarchical structures were constructed too.
| Set number | Zn2+ concentration of growth solution/mM | Growth duration/h | Zn2+ concentration of seed solution/mM | Growth temperature/°C |
|---|---|---|---|---|
| The 1st set | 12.5, 25, 50, 75, 100, 125 | 2.5 | 1 | 90 |
| The 2nd set | 25 | 0.5, 1, 1.5, 2.5, 6 | 1 | 90 |
| The 3rd set | 25 | 2.5 | 0.5, 1, 2, 3, 4, 5 | 90 |
2.3 GOx immobilization on the spirally hierarchical structures
The 3 batches of the spirally hierarchical structures were immersed into 40 mg mL−1 GOx solution for 30 min accordingly, then taken out and dried at room temperature. The samples were rinsed with DI water to remove extra GOx, and then dried at room temperature. Thus the spirally hierarchical structure-based enzymatic electrodes were produced.2.4 Geometric characterization of the spirally hierarchical structures
Based on SEM micrographs and with Image Processing Toolbox of MATLAB, the profiles of the Au cylindrical spirals and the spirally hierarchical structures were extracted, and the geometric parameters (including the actual pitches and the actual outer diameters) of the Au cylindrical spirals and the characteristic parameters (containing roughness Ra, skewness Sk, kurtosis Ku and correlation length ζ) of the surface morphologies of the spirally hierarchical structures were determined.2.5 Electrochemical characterization of the spirally hierarchical structure-based glucose sensors
Cyclic voltammetry was performed in 6 mM glucose solution to investigate the redox abilities of the glucose sensors. The voltage scan range was from −0.2 to +0.8 V, and the scan rate 50 mV s−1. Amperometric response was conducted to determine the sensitivities, linear ranges, limits of detection and Michaelis–Menten constants of the glucose sensors. The bias voltage relative to the reference electrode was +0.8 V. Glucose solution of 100 μL with concentration 0.25 M was intermittently added into 50 mL PBS solution whilst the PBS solution was stirred by a stirrer at 400 rpm.3. Results and discussion
3.1 Geometric characterization of the Au cylindrical spirals and the spirally hierarchical structures
Fig. 1a and b indicates that both the Au cylindrical spiral and the related spirally hierarchical structure are quite compact, uniform, and notably miniaturized. Fig. 1c suggests that the ZnO nanorods of high spatial frequency are reliably synthesized on the Au cylindrical spiral of low spatial frequency in normal direction. Fig. 1d demonstrates that the hexagonal ZnO nanorods are evident and well-aligned, and the surface area of the spirally hierarchical structure greatly increases. It concludes that the process capabilities of the both manually spiralling and hydrothermal synthesis are acceptable. In addition, with the optical fibre core as support, it is more readily to manipulate the Au cylindrical spiral and the spirally hierarchical structure.Fig. 1e and f exhibits the detailed structure of a single ZnO nanorod. Fig. 1e indicates that the as-synthesized ZnO nanorod has hexagonal structure with diameter of about 120 nm. Fig. 1f demonstrates that the lattice spacing (0.52 nm) between two adjacent planes corresponds perfectly to the inter-planar distance between two (002) crystal planes of the wurtzite ZnO. It suggests the preferred growth along the [001] direction of the ZnO nanorod. Both Fig. 1f and its inset demonstrate that the ZnO nanorod is a perfect single crystal with its c-axis as the growing direction.
The blue and red curves in Fig. 1a and b are the extracted profiles of the both Au cylindrical spiral and spirally hierarchical structure. In terms of these curves, the actual pitches and actual outer diameters are determined, referring to Table 2. During the manually spiralling process the Au fibres are elongated to some extent, so their actual diameters become slightly smaller than the nominal one. This further leads to less actual pitches of the both Au cylindrical spirals and spirally hierarchical structures than the respective nominal ones. However the actual pitch of the spirally hierarchical structure is approximately equal to that of the Au cylindrical spiral. This suggests that hydrothermal synthesis changes insignificantly the form of the Au cylindrical spiral. The actual outer diameters of the Au cylindrical spirals are slightly larger than the nominal one. This is ascribed to the negligible spacing between the optical fibre cores and the Au cylindrical spirals. By the way, the average length of the ZnO nanorods is also derived from the actual outer diameters of both the Au cylindrical spiral and related spirally hierarchical structure. All these conclude that the manually spiralling process and hydrothermal synthesis are capable of meeting the geometric requirements for the Au cylindrical spirals and spirally hierarchical structures.
| Pitch/μm | Outer diameter/μm | Length of ZnO nanorods/μm | |||
|---|---|---|---|---|---|
| Nominal | Actual | Nominal | Actual | ||
| Au cylindrical spiral | 30 | 29.47 ± 0.13 | 185 | 186.14 ± 0.24 | — |
| Spirally hierarchical structure | 30 | 29.46 ± 0.08 | >185 | 192.49 ± 0.37 | 3.18 ± 0.31 |
3.2 Characterization of the surface morphologies of the spirally hierarchical structures
The right SEM micrograph in Fig. 2a demonstrates that GOx is immobilized on both the tops and partial flanks of the ZnO nanorods. In Fig. 2b and c the right SEM micrographs exhibit that GOx is immobilized on both the tops and full flanks of the ZnO nanorods. The right SEM micrographs in Fig. 2d and e show that GOx is immobilized on the larger tops and partial flanks of the combined ZnO nanorods, whilst that in Fig. 2f indicates GOx is immobilized only on the tops of the ZnO nanostructures. Different diameters and direction of the ZnO nanorods as well as varied sizes of the pits among the bundles of ZnO nanorods play important roles in dissimilar GOx immobilization on the tops and flanks of the ZnO nanorods/nanostructures.
Likewise the SEM micrographs of the ZnO nanorods synthesized with the 2nd and 3rd sets of the synthesizing parameters as well as related GOx immobilization on these ZnO nanorods are also obtained, referring to Fig. S1 and S3 in the ESI† associated with this article.
Fig. 3 exhibits the XRD patterns of the ZnO nanorods synthesized at different Zn2+ concentration of the growth solution on the Au cylindrically spirals. The diffraction peaks of all XRD patterns are readily indexed to pure ZnO with wurtzite structure (JCPDS no. 36-1451. Space group P63mc (186)). In regard to the ZnO nanorods synthesized at Zn2+ concentration 12.5 mM of the growth solution, the diffraction intensity of (002) peak is slightly stronger than other peaks, suggesting that the orientation of the ZnO nanorods is quite random. As for the ZnO nanorods synthesized at Zn2+ concentration 25, 50, and 75 mM of the growth solution, the diffraction intensities of (002) peaks all are significantly stronger than that of other peaks, meaning that the orientation of the ZnO nanorods is much correlated and perpendicular to the surfaces of the Au cylindrical spirals. In regard to the ZnO nanostructures synthesized at Zn2+ concentration 100 mM of the growth solution, the (002) peak enhances significantly whilst the others roughly keep stable. This indicates that although side-by-side coalescence among neighboring ZnO nanorods occurs, the orientation of the ZnO nanostructures is most uniform and perpendicular to the surface of the Au cylindrical spiral. As for the ZnO nanostructures synthesized at Zn2+ concentration 125 mM of the growth solution, the strength of (002) peak decreases mildly, suggesting that the orientation of the ZnO nanostructures becomes a little random again. The XRD characterization is highly in agreement with the SEM one in Fig. 2.
According to these surface height profiles, the characteristic parameters Ra, Sk, Ku, and ζ of the surface morphology of the spirally hierarchical structures fabricated with 3 sets of the synthesizing parameters are determined, referring to Fig. 5. Fig. 5a illustrates that Ra values of all 3 batches of the spirally hierarchical structures change insignificantly. This suggests that the synthesizing parameters affect slightly the surface properties of the spirally hierarchical structures in vertical direction. As the synthesizing parameters increase ζ values first decrease and then increase, suggesting that the surface properties of the spirally hierarchical structures change significantly along horizontal direction. This is attributed to the changeable sums of the diameters of the ZnO nanorods and the sizes of the pits among the bundles of these ZnO nanorods.
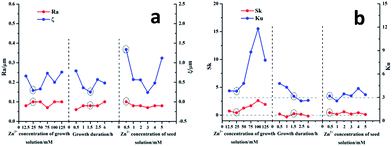 | ||
| Fig. 5 Effects of the synthesizing parameters (3 sets) on the characteristic parameters Ra, ζ (a) and Sk, Ku (b) of the surface morphologies of the spirally hierarchical structures (3 batches). | ||
Fig. 5b demonstrates that for all 3 batches of the spirally hierarchical structures, there do exist an Sk value which approaches to zero (corresponding to that of a Gaussian random rough surface) and a corresponding Ku value which is coarsely equal to 3 (in agreement with that of a Gaussian random rough surface). This indicates that the related spirally hierarchical structures have Gaussian random rough surfaces. In addition, Sk values are positive (negative), indicating that more bumps (pits) appear on the surfaces of the spirally hierarchical structures, whilst Ku values are larger (smaller) than 3, suggesting that the surface heights of the spirally hierarchical structures fluctuate more significantly (insignificantly). This is in agreement with the conclusions from Fig. 2 as well as Fig. S1 and S3 in the ESI† associated with this article.
3.3 Electrochemical characterization of the spirally hierarchical structure-based glucose sensors
Prior to the electrochemical characterization the working temperature of the enzymatic electrode which influences considerably the bioactivity of GOx should be determined first. According to the literatures the optimal working temperature within a testing range 20–65 °C is 50 °C, at which GOx demonstrates the highest bioactivity.26,31 Nevertheless following electrochemical test was still carried out at 25 °C since at room temperature not only the glucose sensor is frequently used but also the evaporation of the testing solution is readily avoided.According to these amperometric responses, the scatter diagrams of the glucose concentration versus the averages of the response currents are obtained and fitted, referring to Fig. 8. In terms of Fig. 8 the sensitivities and linear ranges of the glucose sensors are acquired. With Fig. 7 and 8 the limits of detection are calculated. Based on Lineweaver–Burk equation as well as the reciprocals of the response currents and the glucose concentration which are derived from Fig. 8, the Michaelis–Menten constants are obtained. The calculation details of the aforementioned performance parameters are included in Section B of the ESI† associated with this article.
 | ||
| Fig. 8 Calibration curves of response current versus glucose concentration of the 1st (a), 2nd (b), and 3rd (c) batch of the spirally hierarchical structure-based glucose sensors. | ||
Fig. 9 exhibits the detailed performance of the glucose sensors of 3 batches. Table 3 lists the optimal performance of the glucose sensor of each batch. Albeit the linear ranges and Michaelis–Menten constants of the spirally hierarchical structure-based glucose sensors are as good as that in the literature,24 the sensitivities and limits of detection need to be improved further.22 This is attributed to the ordinary quality of the GOx employed in this paper. Nevertheless, following important findings can still be concluded from Fig. 9. Of all 3 sets of the synthesizing parameters, there exists a desirable one of every set, and the glucose sensor of each batch constructed at this desirable synthesizing parameter has optimal performance. That is, when Zn2+ concentration of the growth solution, the growth duration, and Zn2+ concentration of the seed solution of the 1st, 2nd, and 3rd set synthesizing parameters are 25 mM, 2.5 h, and 0.5 mM respectively, the performance of the corresponding glucose sensor is optimal in comparison with other glucose sensors of that batch. This is in agreement with the conclusion in Section 3.3.1. In comparison Fig. 9 with Fig. 5, apparently the related spirally hierarchical structures fabricated with the desirable synthesizing parameters all have Gaussian random rough surfaces. This gives rise to the largest surface area of the related spirally hierarchical structure, the most effective GOx immobilization of the corresponding enzymatic electrode, and eventually the optimal performance of the related glucose sensor.
| Synthesizing parameters | Synthesizing parameters | Sensitivity (μA mM−1 cm−2) | Linear range (mM) | Limit of detection (μM) | Michaelis–Menten constant (mM) |
|---|---|---|---|---|---|
| The 1st set | 25 mM | 1.02 | 0.019–7.0 | 19 | 5.35 |
| The 2nd set | 1.5 h | 1.38 | 0.011–5.5 | 11 | 2.19 |
| The 3rd set | 0.5 mM | 2.08 | 0.003–9.0 | 3 | 8.28 |
4. Conclusions
With Au fibres manually spiralled around optical fibre cores and ZnO nanorods hydrothermally synthesized on the Au cylindrical spirals, the spirally hierarchical structures were fabricated as batch and effectively miniaturized. GOx was immobilized on these ZnO nanorods and the spirally hierarchical structure-based glucose enzymatic electrodes were produced. Since the optical fibre cores were employed as support, the manipulation of the Au cylindrical spirals, the spirally hierarchical structures, and the enzymatic electrodes was significantly improved. Quantitative characterizations of the surface morphologies and the performance of the glucose sensors indicate that when Zn2+ concentration of the growth solution, the growth duration, and Zn2+ concentration of the seed solution of the 3 sets of the synthesizing parameters are set to be 25 mM, 1.5 h, and 0.5 mM respectively, the related spirally hierarchical structures all have Gaussian random rough surfaces. This results in the largest surface area of the related spirally hierarchical structure, the most effective GOx immobilization of the corresponding enzymatic electrode, and the optimal performance of the related glucose sensor of each batch. The results benefit not only the batch production but also standardization of other hierarchical structure-based glucose sensors.Acknowledgements
We would like to acknowledge the financial support from the NSFC Major Research Plan on Nano-manufacturing (No. 51075324, 91323303), the Key Science and Technology Program of Shaanxi Province (No. 2015GY117), the National Key Scientific Instrument and Equipment Development Projects of China (No. 2012YQ03026101), the National Key Basic Research Program of China (No. 2015CB057400), and the 111 Project (No. B12016).Notes and references
- C. Chen, Q. J. Xie, D. W. Yang, H. L. Xiao, Y. C. Fu, Y. M. Tan and S. Z. Yao, RSC Adv., 2013, 3, 4473–4491 RSC.
- A. A. Ansari, A. Kaushik, P. R. Solanki and B. D. Malhotra, Electrochem. Commun., 2008, 10, 1246–1249 CrossRef CAS PubMed.
- Y. S. Zou, L. L. He, K. Dou, S. L. Wang, P. L. Ke and A. Y. Wang, RSC Adv., 2014, 4, 58349–58356 RSC.
- G. C. Gil, Y. J. Kim and M. B. Gu, Biosens. Bioelectron., 2002, 17, 427–432 CrossRef CAS.
- D. W. Pan, J. H. Chen, S. Z. Yao, L. H. Nie, J. J. Xia and W. Y. Tao, Sens. Actuators, B, 2005, 104, 68–74 CrossRef CAS PubMed.
- S. M. Usman Ali, O. Nur, M. Willander and B. Danielsson, Sens. Actuators, B, 2010, 145, 869–874 CrossRef CAS PubMed.
- D. Wilke, H. Müller and N. Kolytsheva, Fresenius. J. Anal. Chem., 1997, 357, 534–538 CrossRef CAS.
- F. Dehghan Nayeri, E. Asl Soleimani and F. Salehi, Renewable Energy, 2013, 60, 246–255 CrossRef PubMed.
- J. R. Anusha, H. J. Kim, A. T. Fleming, S. J. Das, K. H. Yu, B. C. Kim and C. J. Raj, Sens. Actuators, B, 2014, 202, 827–833 CrossRef CAS PubMed.
- A. Wei, X. W. Sun, J. X. Wang, Y. Lei, X. P. Cai, C. M. Li, Z. L. Dong and W. Huang, Appl. Phys. Lett., 2006, 89, 123902 CrossRef PubMed.
- S. Ishizuka, K. Suzuki, Y. Okamoto, M. Yanagita, T. Sakurai, K. Akimoto, N. Fujiwara, H. Kobayashi, K. Matsubara and S. Niki, Phys. Status Solidi C, 2004, 1, 1067–1070 CrossRef PubMed.
- W. X. Jing, H. Qi, L. L. Niu, Z. D. Jiang, B. Wang, L. J. Chen, F. Zhou and Y. L. Zhao, Phys. E, 2014, 56, 196–204 CrossRef CAS PubMed.
- A. Janotti and C. G. van de Walle, Appl. Phys. Lett., 2005, 87, 122102 CrossRef PubMed.
- M. Meshki, M. Behpour and S. Masoum, J. Electroanal. Chem., 2015, 740, 1–7 CrossRef CAS PubMed.
- D. M. Bagnall, Y. F. Chen, Z. Zhu, T. Yao, S. Koyama, M. Y. Shen and T. Goto, Appl. Phys. Lett., 1997, 70, 2230–2232 CrossRef CAS PubMed.
- A. J. Cheng, Y. H. Tzeng, Y. Zhou, M. Park, T. H. Wu, C. Shannon, D. Wang and W. Lee, Appl. Phys. Lett., 2008, 92, 092113 CrossRef PubMed.
- S. S. Lin and J. L. Huang, Surf. Coat. Technol., 2004, 185, 222–227 CrossRef CAS PubMed.
- L. F. Xu, Y. Guo, Q. Liao, J. P. Zhang and D. S. Xu, J. Phys. Chem. B, 2005, 109, 13519–13522 CrossRef CAS PubMed.
- S. Baruah and J. Dutta, Sci. Technol. Adv. Mater., 2009, 10, 013001 CrossRef.
- D. Polsongkram, P. Chamninok, S. Pukird, L. Chow, O. Lupan, G. Chai, H. Khallaf, S. Park and A. Schulte, Phys. B, 2008, 403, 3713–3717 CrossRef CAS PubMed.
- J. J. Dong, C. Y. Zhen, H. Y. Hao, J. Xing, Z. L. Zhang, Z. Y. Zheng and X. W. Zhang, Nanoscale Res. Lett., 2013, 8, 378 CrossRef PubMed.
- R. Ahmad, N. Tripathy, J. H. Kim and Y. B. Hahn, Sens. Actuators, B, 2012, 174, 195–201 CrossRef CAS PubMed.
- J. Y. Kim, S. Y. Jo, G. J. Sun, A. Katoch, S. W. Choi and S. S. Kim, Sens. Actuators, B, 2014, 192, 216–220 CrossRef CAS PubMed.
- Y. L. Zhai, S. Y. Zhai, G. F. Chen, K. Zhang, Q. L. Yue, L. Wang, J. F. Liu and J. B. Jia, J. Anal. Chem., 2011, 656, 198–205 CAS.
- W. X. Jing, F. Zhou, Y. Y. Cheng, H. Qi, L. J. Chen, Z. D. Jiang and B. Wang, Chin. J. Anal. Chem., 2014, 42, 1077–1082 CAS.
- T. Kong, Y. Chen, Y. P. Ye, K. Zhang, Z. X. Wang and X. P. Wang, Sens. Actuators, B, 2009, 138, 344–350 CrossRef CAS PubMed.
- S. M. Usman Ali, Z. H. Ibupoto, S. Salman, O. Nur, M. Willander and B. Danielsson, Sens. Actuators, B, 2011, 160, 637–643 CrossRef PubMed.
- G. Zhong, A. Kalam, A. S. Al-Shihri, Q. M. Su, J. Li and G. H. Du, Mater. Res. Bull., 2012, 47, 1467–1470 CrossRef CAS PubMed.
- Z. S. Hu, G. Oskam and P. C. Searson, J. Colloid Interface Sci., 2003, 263, 454–460 CrossRef CAS.
- P. Suresh Kumar, P. Paik, A. Dhayal Raj, D. Mangalaraj, D. Nataraj, A. Gedanken and S. Ramakrishna, Appl. Surf. Sci., 2012, 258, 6765–6771 CrossRef PubMed.
- X. F. Chu, X. H. Zhu, Y. P. Dong, T. Y. Chen, M. F. Ye and W. Q. Sun, J. Electroanal. Chem., 2012, 676, 20–26 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra15346h |
| This journal is © The Royal Society of Chemistry 2015 |







